“Safe and effective”. That’s the phrase that tumbles from the lips of medical professionals, corporate media talking heads, politicians and health bureaucrats whenever the topic of vaccines – and most especially the experimental injections labelled as “COVID-19 vaccines” comes up.
Like a mantra, “safe and effective” is repeated endlessly with the intention to induce a state of calm and banish from the mind all thoughts apart from the intended focal point – the notion that the vaccine is what you want… the vaccine is desirable… the vaccine is your saviour.
But how do we know that any pharmaceutical product is “safe and effective”? The answer is, we rely on properly-conducted clinical trials, and vigilant post-marketing surveillance.
As discussed at length in my previous articles How many people have COVID-19 injections harmed? Part 1 and To jab or not to jab? That is the question, post-marketing surveillance in Australia and many other countries is incredibly weak, depriving us of the ability to assess how safe the COVID-19 injections are in the real world setting rather than in the “perfect patients” selected to participate in clinical trials.
On the effectiveness front, a crop of recent scientific papers has cast significant doubt on the real-world efficacy of these novel products. Let’s examine two of these papers, in some detail:
1. Shedding of infectious SARS-CoV-2 despite vaccination
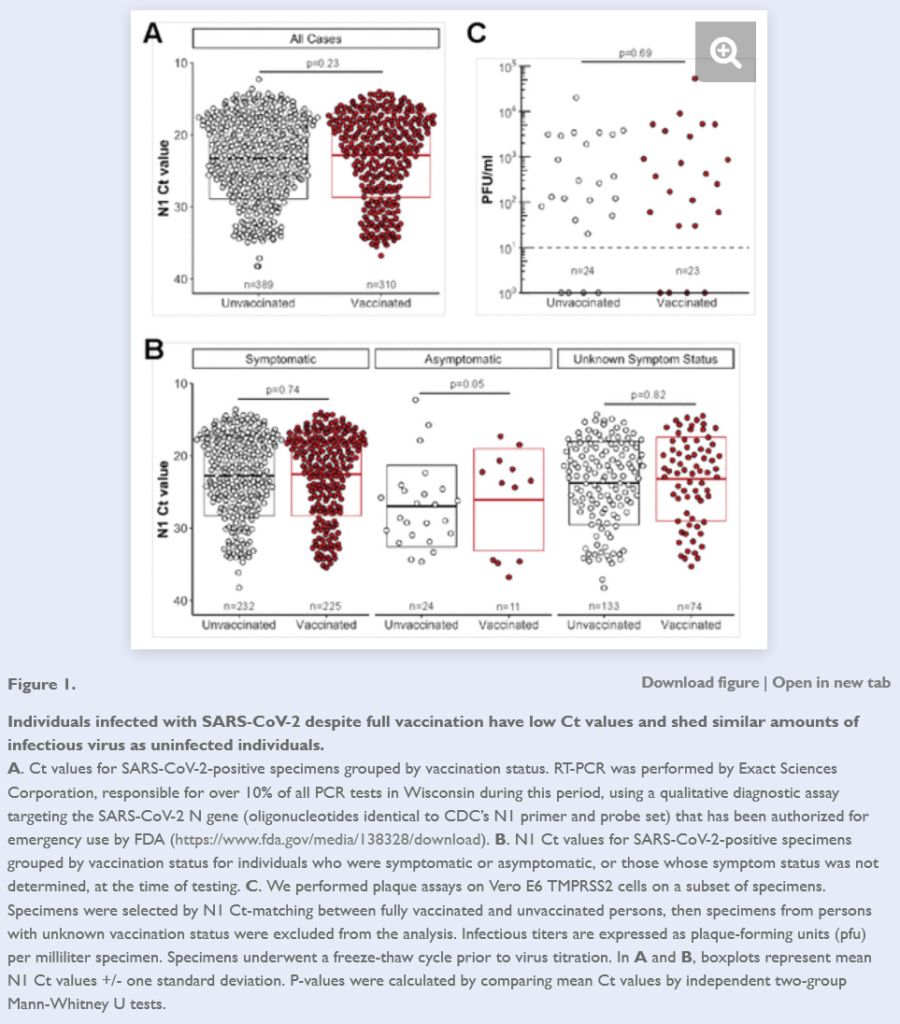
This pre-print study, authored by scientists from the US Centers for Disease Control and Prevention (CDC) and a county public health department in the state of Wisconsin, compared cycle threshold values (an indicator of the quantity of virus detected in a sample) in almost 700 nasal swab specimens from both unvaccinated and fully vaccinated individuals who tested positive on rt-PCR for SARS-CoV-2.
They found Ct values under 25 (indicating a high viral load – that is, larger quantities of virus, and a greater risk of shedding infectious virus) in 68% of fully vaccinated people vs 63% of unvaccinated people.
Furthermore, Ct values under 25 were detected in 82% of fully vaccinated people who had no symptoms of infection at the time of testing, compared to only 29% of asymptomatic unvaccinated people.
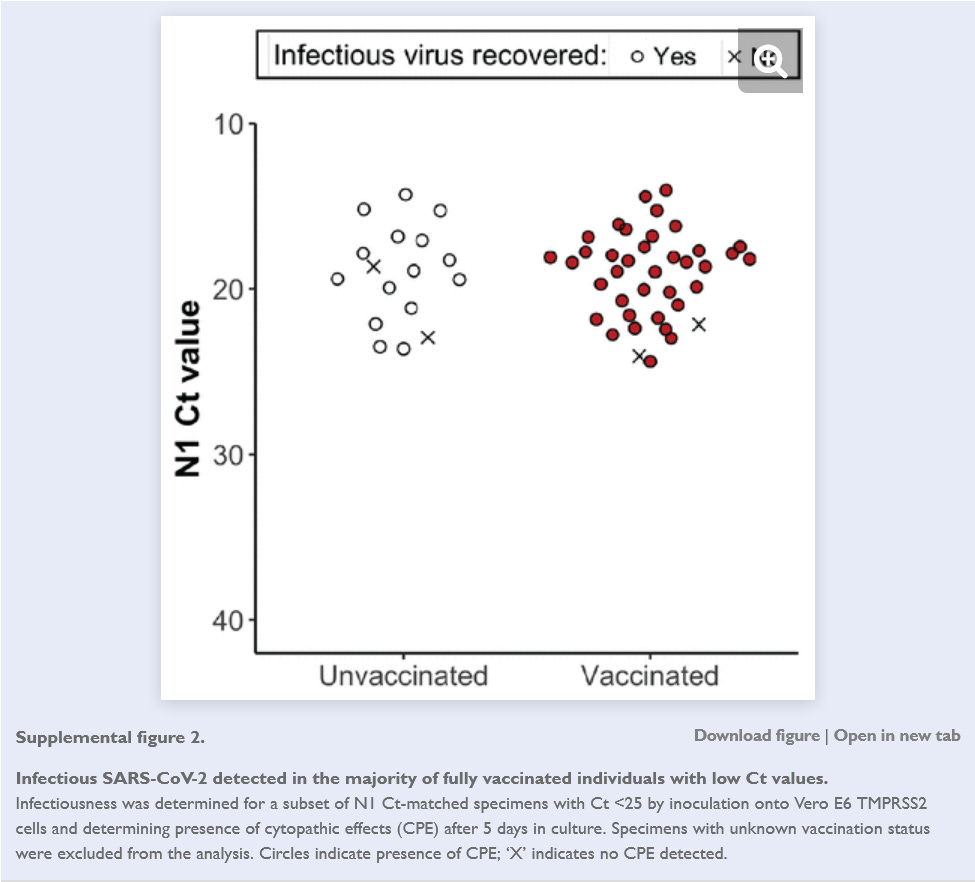
They then tested a subset of swab specimens and found infectious SARS-CoV-2 in 95% of vaccinated individuals vs 88% of the unvaccinated.
And finally, they performed a test to measure the amount of infectious virus present in low Ct specimens, and found no difference in vaccinated vs unvaccinated individuals.
In other words, this study found that vaccinated people were more likely to be shedding infectious virus than unvaccinated people and that they were much more likely than to be shedding infectious virus than unvaccinated people when they had no indication that they were infected.
To put it bluntly, fully vaccinated people are the perfect superspreaders. Unvaccinated people who become infected with SARS-CoV-2 and have enough virus present to infect other people, are far more likely to have symptoms of illness that prompt them to stay home and avoid contact with other people, while infected vaccinated people are out and about, shedding infectious virus in their saliva while they interact with others.
2. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study
This study, published in Lancet, investigated the transmission of the Delta variant of SARS-CoV-s between people who lived in the same household.
The researchers found that:
Household contacts exposed to a fully vaccinated person infected with the Delta variant (known in epidemiology as the “index case”) had a 25% risk of becoming infected themselves, while household contacts exposed to an unvaccinated index case had a 23% risk.
38% of fully vaccinated index cases gave rise to secondary transmission (i.e. they infected a household member), compared to 41% of unvaccinated index cases.
Fully vaccinated individuals who were household contacts of an index case infected with the Delta variant of SARS-CoV-2 had a 25% risk of becoming infected themselves, compared to a 38% risk in unvaccinated individuals. The difference did not reach statistical significance.
39% of infections in fully vaccinated household contacts arose from fully vaccinated index cases, with genomic and virological analysis confirming the linkage.
Peak viral load (that is, the maximum amount of virus present during the period of infection) did not differ by vaccination status.
The effectiveness of COVID-19 vaccination at preventing infection (regardless of symptoms) with the Delta strain in the household setting was a mere 34%.
Susceptibility to infection increases over time, beginning as soon as 2–3 months after vaccination, indicating rapid waning of protective immunity.
The proportion of asymptomatic cases of Delta infection was the same regardless of vaccination status (fully vaccinated, partially vaccinated, or unvaccinated).
Viral load declined at a slightly faster rate in vaccinated individuals with a breakthrough Delta infection than in unvaccinated individuals, but the impact on household contacts’ risk of infection was minimal, as the authors admitted: “The modest scale of differences in viral kinetics between fully vaccinated and unvaccinated individuals with delta infection might explain the relatively high rates of transmission seen from vaccinated delta index cases in our study.”
Based on their detailed analysis of the transmission of SARS-CoV-2 within households, the authors flatly concluded that
“Fully vaccinated individuals with breakthrough infections have peak viral load similar to unvaccinated cases and can efficiently transmit infection in household settings, including to fully vaccinated contacts.”
But hold on a minute. Didn’t Pfizer, Moderna et al variously claim that their products were “more than 90% effective“, then “more than 94% effective“, and then “95% effective“? And weren’t they granted provisional approval or emergency use authorisation largely on the basis of those efficacy claims? And didn’t the media lackeys of the vaccine manufacturers endlessly spruik those claims, long before the injection roll-out began?
Just as an aside, I wonder if the media’s enthusiasm for the jabs has anything to do with this:
Those claims of effectiveness were based on the results of clinical trials sponsored by the pharmaceutical companies that produce the injections. But pharma companies don’t actually conduct such clinical trials themselves. Instead, the day-to-day business of recruiting trial participants, administering the study intervention, monitoring participants for adverse effects and gathering data on study outcomes (such as infection rates) is mostly conducted by privately owned contract research organisations.
And now, whistleblowers have stepped forward to describe the deeply concerning goings-on at one such privately owned research organisation, Ventavia Research Group.
Ventavia conducted studies on Pfizer’s COVID-19 injection at several sites in Texas last year, as part of the phase 3 clinical trial that the pharma giant used to gain emergency use authorisation/provisional registration for the product.
In an investigative piece for The BMJ titled ‘Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial‘, journalist Paul Thacker recounts the whistleblower testimony of Brook Jackson.
Jackson is a trained clinical trial auditor with over 15 years’ experience in clinical research coordination and management. She served as a regional director for Ventavia for 2 weeks in September 2020, with responsibility for overseeing over 1000 participants in Pfizer’s phase 3 clinical trial, at three Texas study sites.
During the brief period that she worked for Ventavia, Ms Jackson observed multiple instances of poor laboratory management, patient safety concerns, and data integrity issues, including:
Employment of inadequately trained vaccinators
Lack of appropriate monitoring of participants immediately after injection (participants were placed in a hallway with no supervision by clinical staff)
Falsification of data
Unblinding of patients
Lack of timely follow-up of patients who experienced adverse events
Protocol deviations not reported
Vaccines not stored at proper temperatures
Laboratory specimens mislabelled, and
Targeting of Ventavia staff for reporting these types of problems.
Jackson repeatedly notified her superiors of the serious issues she had observed, but her concerns were blown off. Finally, she phoned the FDA on 25 September 2020 to warn about unsound practices in Ventavia-managed sites of Pfizer’s clinical trial, and followed up with an email detailing her concerns.
Ventavia fired her the same day, which raises the suspicion that someone at FDA may have tipped Ventavia off about Jackson’s complaint. If true, this would constitute appalling malfeasance.
Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails which verify the allegations she has made against Ventavia.
You can watch an interview with Jackson in which some of those documents are shown, here:
Under condition of anonymity, two former Ventavia employees have confirmed the substance of Jackson’s complaint to The BMJ.
One, a veteran of over four dozen clinical trials including many large ones, commented,
“I’ve never had to do what they were asking me to do, ever. It just seemed like something a little different from normal—the things that were allowed and expected.”
One important instance: Ventavia lacked the staffing to swab all trial participants who reported COVID-19-like symptoms, to test for infection. Hence there is a high probability that many infections were missed.
Prevention of symptomatic COVID-19 was the primary end-point of the trial, so missing cases of infections would obviously call into question Pfizer’s conclusion that its product was effective.
When this piece of information is put together with Brook Jackson’s allegation of wide-spread unblinding of participants – that is, trial staff who were not meant to know whether participants had received the Pfizer injection or a placebo did in fact know this crucial information, the possibility emerges that trial staff may have deliberately avoided testing participants who developed COVID-19 symptoms after receiving the Pfizer injection, skewing the trial results in Pfizer’s favour.
The other anonymous former Ventavia employee “described an environment at Ventavia unlike any she had experienced in her 20 years doing research.” She also revealed that Pfizer conducted an audit of Ventavia shortly after Brook Jackson was fired.
Unlike Jackson, it seems Pfizer was not concerned by Ventavia’s acts of omission and commission. In fact, they hired the research company as a subcontractor on three other clinical trials of their COVID-19 injection (in children and young adults, pregnant women, and a booster dose), as well as an RSV vaccine trial.
The FDA’s recent decision to grant emergency use authorisation for the Pfizer injection in 5-11 year olds was based on the first of these clinical trials, including the Ventavia-administered trial sites.
And if you think that this must have been a one-off, and Ventavia was the only contract research organisation engaging in dodgy activities during the phase 3 trial of the Pfizer COVID-19 injection, I have a bridge to sell you.
So next time you hear someone regurgitating the shibboleth that any of these rushed-to-market experimental COVID-19 injections are “safe and effective”, remember that the Big Lie can only fool people who refuse to believe that anyone could tell a lie on such a colossal scale, and then keep telling it, over and over, without any shame. You’d better believe that there are people who can.
P.S. Dr David Healy knows a thing or two about the way Pfizer does business. You can watch his testimony to Senator Ron Johnson’s roundtable discussion on COVID-19 injection injuries here (1 hr 24 mins, and 2 hr 21). In between, you can have your heart ripped right out of your chest and torn into a thousand tiny pieces by hearing all the personal stories of both children and adults who have been catastrophically injured by these products. You’re welcome.






What are your suggestions for stopping the spread in both vaccinated and unvaccinated people ? In the state I live in (WA) we had quick hard lockdowns and used masks etc and had relative freedom in between lockdowns although we couldn't really travel.
Those measures are coming to an end and it does seem to be the unvaccinated who are dying of Delta more often than the vaccinated. How can more deaths be prevented ?