I have had more requests to write an article about the Novavax COVID-19 vaccine1 than about any other topic during the entire manufactured crisis of the past two years. I’ve been holding back until I felt I had enough information to make it worth your while to read. I’ll be adding to this article as more data come in, so check back in on it from time to time.
As one of my correspondents put it, the Novavax vaccine is “being subtly pushed by doctors to get the people who for various reasons did not want the usual culprit vaccines [i.e. the mRNA and viral vector injections, which many resisted due to concerns about novel technologies and adverse reactions] but who may be persuaded that this one is different”.
It’s a strange state of affairs when a product’s marketing angle is “Not nearly as dangerous as its competitors!”… but here we are.
So just what is the Novavax shot, what technology is used to produce it, who is making it, what’s in it, is it safe, and is it effective at reducing the risk of infection with SARS-CoV-2 and the development of COVID-19?
What is the Novavax injection?
The Novavax vaccine, officially designated “SARS-CoV-2 rS (NVX-CoV-2373)”, is branded in Australia as Nuvaxovid, for reasons presumably best known to its sponsors who no doubt spent a small fortune on focus groups to pick a catchy name for their product.
Unlike traditional vaccines against viral diseases, which are produced by growing viruses in chicken eggs, then harvesting the virus from the eggs and either attenuating (weakening) or inactivating (killing) it, Nuvaxovid uses recombinant DNA technology.
Recombinant technology is a relative newcomer to the vaccine manufacturing space; the US Food and Drug Administration (FDA) approved the first recombinant influenza vaccine in 2013.
Recombinant vaccines are created synthetically, by inserting a gene that codes for a viral protein that triggers the human immune system – known as an antigen – into a baculovirus (a virus that infects invertebrates) to produce a “recombinant” baculovirus. Host cells are then deliberately infected with the recombinant baculovirus, forcing them to make many copies of the viral antigen. The antigen is then collected, purified, and combined with chemicals that ramp up the immune system’s response to antigens – known as adjuvants – to produce a vaccine.
In the case of the Novavax shot, the viral antigen is a modified version of the spike protein of the original Wuhan strain of SARS-CoV-2, the host cells are derived from the immature ovaries of the pupae of fall armyworm moths (Spodoptera frugiperda), and the adjuvant is Matrix-M, which is a combination of two saponins (soap-like substances) derived from the sap of the soapbark tree (Quillaja saponaria) encased in cholesterol nanoparticles to reduce their toxicity to human cells. The spike proteins harvested from the moth cells are assembled onto synthetic lipid nanoparticles, each displaying up to 14 spike proteins.
Who makes the Novavax vaccine?
Novavax, a company based in the US state of Maryland, describes itself as “a biotechnology company that promotes improved health globally through the discovery, development and commercialization of innovative vaccines to prevent serious infectious diseases.”
Novavax claims that they have “more than a decade of experience contending with some of the world’s most devastating diseases, including COVID-19, seasonal influenza, RSV, Ebola, MERS, and SARS”. Yet, just like mRNA injection producer Moderna, Novavax had never brought any of its candidate vaccines to market until the unprecedented global response to the COVID-19 crisis helped to “blow the system up”, smashing barriers to the acceptance of novel vaccine technologies in just the way that New Yorker staff writer, Michael Specter, salivated over in the October 2019 Milken Institute gabfest on developing a universal flu vaccine (start watching around 8:10 for Specter’s spiel):
How does a for-profit organisation persist since 1987, continuing to pay staff and pull in funding for its research and development, without managing to produce a single marketable product? I guess it pays to have friends in the right places.
And Novavax certainly appears to have the right kind of friends, managing to attract funding from the Bill Gates-founded Coalition for Epidemic Preparedness Innovations (CEPI) to the tune of up to US$388 million for its COVID-19 vaccine candidate, along with a cool US$1.6 billion from the US government-sponsored Operation Warp Speed program to fast-track the development of vaccines for COVID-19. The US Department of Defense awarded Novavax a $70 million contract for producing their vaccine in June 2020, despite the Phase 1 (preliminary immunogenicity and safety) trial having only begun in May 2020, with results not reported until July 2020 – a month after the contract was inked.
What’s in the Novavax vaccine?
In addition to the bioengineered spike protein, the TGA Product Information document lists the following excipients:
Dibasic sodium phosphate heptahydrate
Monobasic sodium phosphate monohydrate
Sodium chloride
Polysorbate 80
Sodium hydroxide (for adjustment of pH)
Hydrochloric acid (for adjustment of pH)
Water for Injections
Adjuvant (Matrix M)
Quillaja saponaria saponins fraction A
Quillaja saponaria saponins fraction C
Cholesterol
Phosphatidyl choline
Monobasic potassium phosphate
Potassium chloride
While most of these ingredients are quite innocuous, polysorbate 80 is associated with an increased risk of colorectal cancer when consumed as a food additive, and is linked to hypersensitivity systemic reactions (such as anaphylaxis), kidney and liver toxicity and increased susceptibility of cells to oxidative stress when used in drugs that enter the bloodstream. Polysorbate 80 also facilitates the transport of drugs across the blood brain barrier, the structure which hinders the entry of most substances into the delicate neural tissue that comprises the brain. The implications of this for a drug that contains a spike protein which is known to cause inflammation within the brain and suspected to be responsible for the fatigue and neuropsychiatric symptoms that characterise both acute and long COVID, are deeply concerning. Does the presence of polysorbate 80 in the Novavax shot increase uptake of spike protein into the brain? And if so, how does this affect the risk of stroke, vascular dementia, and cognitive dysfunction?
Updates:
A correspondent pointed out to me that the presence of lipid nanoparticles in the Novavax vaccine also increases the uptake of spike protein into fat-rich tissues, such as the brain. Are there synergistic effects of these lipid nanoparticles with polysorbate 80, that may result in even more spike protein entering the brain than from either substance alone?
Fellow Substacker, A Midwestern Doctor, provided a link to this article, which flagged the known hazards of injecting even minute amounts of cholesterol - namely, induction of anaphylaxis and autoimmune disease:
Following this lead, I found the book referred to in the article, Vaccine A, on Archive.org.
If you enter “cholesterol'“ into the Search Inside field, you’ll find 27 results, and you can page through them to trace the story of how researchers discovered that injecting cholesterol into the body induces the formation of antibodies against cholesterol, and that these antibodies activate the complement system, which (although a crucial component of the immune response to infection) can cause damaging “friendly fire” attacks on our own tissues. Autoantibodies to cholesterol also trigger blood clots and could be a causal factor in atherosclerosis, the pathology underlying heart attacks and ischaemic strokes. Has any testing been done to check whether such antibodies are formed in individuals who receive vaccines that use cholesterol-containing nanoparticles?
These are the types of questions that one would expect to be answered in preclinical studies, that is, studies performed on laboratory animals (sorry, vegans, but if you’re going to take pharmaceutical products, you have to know that vast numbers of rodents, primates and other non-human animals are tortured and sacrificed by this industry every year). So…
What did the studies on Nuvaxovid show?
The preclinical Australian Product Information document for Nuvaxovid states only that genotoxicity tests (i.e. studies to detect damage to chromosomes) were carried out on only the Matrix-M adjuvant, not on the whole product, and that
“Carcinogenicity studies were not performed. The components of the vaccine are not expected to have carcinogenic potential.”
“Not expected to have carcinogenic potential”? What about the demonstrated colon cancer-accelerating effect of polysorbate 80? Sure, carcinogenesis has only been demonstrated so far with oral dosing of polysorbate 80, but the mechanism of action is promotion of inflammation, which is known to increase the risk of all manner of cancers. And since the spike protein itself triggers inflammation, there may be a synergistic effect on cancer promotion when polysorbate 80 is delivered in combination with spike protein.
Clinical trials – Phase 1 & 2
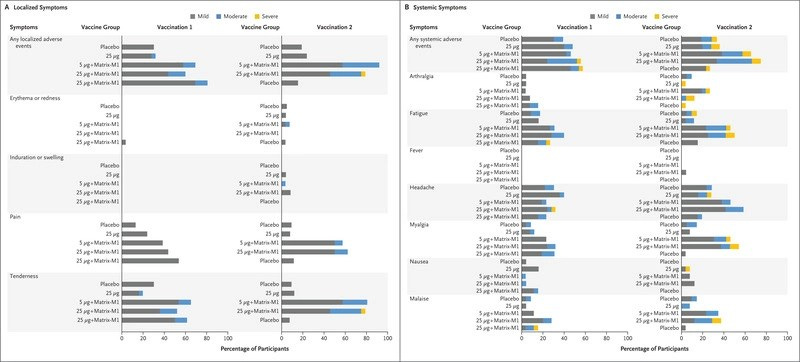
Moving onto studies in humans (clinical trials), a combined Phase 1/2 trial to evaluate safety and immunogenicity in 131 healthy Australian adults concluded that the product – which was tested in a number of different formulations – induced higher antibody levels than infection with SARS-CoV-2, as well as a robust T cell response, and had an acceptable safety profile, with no serious adverse events. Notably, however, only those who received the vaccine reported adverse reactions ranked as “severe” (i.e. none occurred in the placebo group) and severe reactions were more common after the second shot than after the first:
A larger Phase 2 trial enrolling 1288 participants from the US and Australia once again found a stronger neutralising antibody response after vaccination than in convalescent sera – that is, the antibodies from vaccinated individuals were better at killing SARS-CoV-2 than antibodies from people who had recovered from infection, but notably, both were tested against the original Wuhan version of the virus (“wild type”) which was no longer in circulation at the time the study took place, having long since been replaced by a series of variants.
Once again, moderate to severe adverse reactions were more frequent after the second shot than the first. They also occurred more frequently in the higher-dose version of the vaccine. One vaccine recipient developed myocarditis three days after the second dose, and was hospitalised.
Clinical trials – Phase 3
1. The UK study
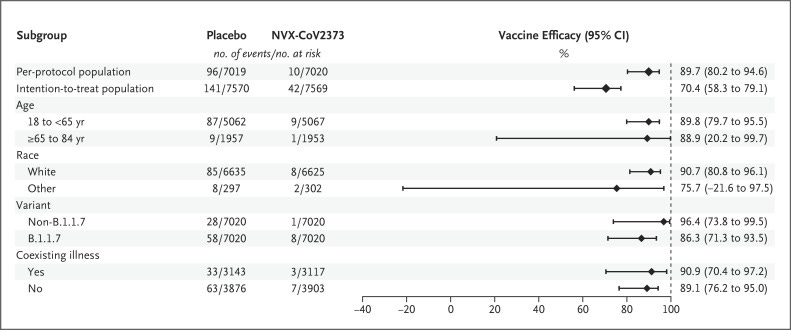
The first Phase 3 trial to be published, in June 2021, randomised over 15 000 UK adults to receive either two doses of the lower dose version of the Novavax vaccine, or saline placebo injection, three weeks apart. The triumphant conclusion of the study was that the two-dose regimen “conferred 89.7% protection against SARS-CoV-2 infection and showed high efficacy against the B.1.1.7 [Alpha] variant”. But what does this actually mean?
The primary end point of the trial was the “first occurrence of virologically confirmed symptomatic mild, moderate, or severe Covid-19 with onset at least 7 days after the second dose among participants who were seronegative at baseline, as determined by the results of testing for anti–nucleocapsid antibody. Symptomatic Covid-19 was defined according to the criteria of the FDA.”
In other words, anyone who reported even the mildest of generic respiratory viral symptoms such as cough, headache or fever and who returned a positive PCR test was declared to “have COVID-19”, even though PCR is not suitable for diagnosing infection. The cycle threshold used for PCR testing was not specified.
Furthermore, there is a 40-100% increased risk of SARS-CoV-2 infection for two weeks after the first Pfizer COVID-19 injection, but since the efficacy calculations in the UK Novavax trial excluded all “cases” of COVID-19 that occurred in the 4-week period between the first shot and 1 week after the second shot, it is not known whether the same negative efficacy occurs with the Novavax vaccine.
What is known is that out of the two deaths related to COVID-19 that were reported during the trial (one in the vaccine group and one in the placebo group), “the death in the vaccine group occurred in a 53-year-old man in whom Covid-19 symptoms developed 7 days after the first dose; he was subsequently admitted to the ICU for treatment of respiratory failure from Covid-19 pneumonia and died 15 days after vaccine administration.”
Just in case you missed it, the Novavax vaccine did not reduce the risk of dying of COVID-19. Furthermore, while the authors made much of the fact that the five reported cases of “severe COVID” all occurred in the placebo group, only one of these five required hospital treatment. Three of these “severe” cases attended the emergency department but were not admitted, while the fifth was treated at home.
And oddly, the Novavax shot showed no statistically significant benefit in non-white participants:
So, this much-touted trial demonstrated that Nuvaxovid
Did not prevent death from COVID-19 (1 death each in the placebo and vaccine group)
Did not prevent hospitalisation (1 hospitalisation for COVID-19 in the placebo group, one for immune-mediated myocarditis in the vaccine group)
Reduced the absolute risk of developing any symptom of COVID-19 in the first three months after shot #2 + one week by 1.23% (from 1.37% in the placebo group to 0.14% in the vaccine group).
The trial did not assess whether the vaccine blocked transmission of SARS-CoV-2.
2. The US/Mexico study
Just under 30 000 US and Mexican adults aged over 18 were recruited for this trial, which took place in the first half of 2021, when the Alpha variant was still dominant in North America. Vaccine efficacy was calculated at 90.4%, using the same criteria as in the UK trial: any symptom of COVID-19, along with a positive PCR test, occurring at least 7 days after the second dose.
In all, 14 such “cases” of COVID-19 occurred within the roughly 2 month follow-up time in the 17 312 people who received two doses of the Novavax vaccine per protocol, vs 63 “cases” in the 8140 placebo recipients.
10 moderate and 4 severe cases of COVID-19 were reported in the placebo group (although none appear to have required hospitalisation), and none in the vaccine group. No deaths from COVID-19 occurred in either group.
Efficacy in Latinos was lower than in either non-Latino white, or black people:
Once again, adverse events were reported by more people who received the vaccine than the placebo, and were more frequent after the second vaccine dose. Severe local reactions after dose #2 occurred in less than 1% of placebo recipients vs 6.7% of vaccine recipients. Severe systemic reactions occurred in 2.1% of placebo recipients after dose #2 vs 12.1% of vaccine recipients.
In summary, the US/Mexico trial found that Nuvaxovid:
Did not prevent death or hospitalisation from COVID-19.
Reduced the absolute risk of developing any symptom of COVID-19 in the first two months after shot #2 + one week by 0.69% (from 0.77% in the placebo group to 0.08% in the vaccine group).
Resulted in significantly increased risk of severe local and systemic reactions compared to placebo, particularly after the second dose.
As with the UK trial, this study did not assess whether the vaccine prevents transmission of SARS-CoV-2.
3. The South Africa study
In this study, 4387 South African adults (94% HIV-negative and 6% HIV-positive) received two doses of either Nuvaxovid or placebo, during a period in which the Beta variant of SARS-CoV-2 was dominant. Using the same criteria as for the UK and US trials, vaccine efficacy was found to be just 49.4% overall, and 60.1% in HIV-negative participants. In roughly 60 days of follow-up, symptomatic COVID-19 was observed in 15 participants out of the 1357 in the vaccine group who completed the study per protocol, and in 29 participants out of the 1327 in the placebo group.
No COVID-related deaths or hospitalisations occurred in either the vaccine or placebo group, and only one of the 29 “cases” of COVID-19 that occurred in the placebo group was rated as severe.
Medically attended adverse events and serious adverse events occurred more often in the vaccine group than in the placebo group (13 vs. 6 medically attended adverse events and 2 vs. 1 serious adverse events).
In summary, the South African trial found that Nuvaxovid:
Did not reduce the risk of hospitalisation or death from COVID-19.
Reduced the absolute risk of developing any symptom of COVID-19 in the first two months after shot #2 + one week by 1.08% (from 2.19% in the placebo group to 1.11% in the vaccine group).
Doubled the risk of experiencing a medically attended adverse event or serious event.
And, as with the UK and US/Mexico trials, the South African study did not assess whether the vaccine presents transmission of SARS-CoV-2.
The TGA granted provisional approval to Nuvaxovid on these data????
You’ll forgive me for being just a little underwhelmed by the efficacy data on the Novavax shot. The three flagship trials on which TGA granted provisional approval for Nuvaxovid show no mortality benefit and no reduction in hospitalisation risk. They also don’t demonstrate any community benefit, because prevention of transmission of SARS-CoV-2 wasn’t an endpoint.
The trade-off for the 0.69-1.23% reduction in your risk of developing any respiratory tract symptom with a positive PCR test for SARS-CoV-2 is a significantly elevated risk of severe local and systemic adverse reactions.
In addition to the two myocarditis cases identified in the Phase 1/2 trial and the UK trial, the European Medicine Agency’s assessment of Nuvaxovid identified a third case, which occurred in a participant in the placebo group who was given the vaccine after the follow-up period was completed.
Update: Just to make the preceding point clear, as for all the other COVID-19 vaccine trials, the control group in the Novavax Phase 3 trials was abolished by offering all participants who received placebo the vaccine, after the 2-3 month observation period had elapsed.
Hypertension (high blood pressure) was also identified as an adverse reaction to Nuvaxovid; a pooled safety analysis found 4 serious adverse events of hypertension as well as 13 severe cases of hypertension. They also noted more serious adverse events of prostate cancer (5 in the vaccine group vs 0 in the placebo group) and stroke (7 vs 1).
ATAGI’s advice contradicts the TGA
The conflict of interest-ridden Australian Technical Advisory Group on Immunisation (ATAGI) issued a statement on the use of Novavax COVID-19 vaccine (Nuvaxovid) which contradicts the product information document issued by TGA.
For example, TGA takes a cautious approach to use in pregnancy and breastfeeding:
ATAGI, on the other hand, throws caution to the wind:
TGA acknowledges that there is scant data on efficacy and safety in immunocompromised people, and none on “mixing and matching” Nuvaxovid with other COVID-19 vaccines:
ATAGI makes no such acknowledgement of the lack of data and has no qualms whatsoever about tacking a dose of Nuvaxovid onto a primary course of a different type of COVID-19 vaccine:
What are we to make of this conflicting guidance – and why is ATAGI permitted to issue recommendations that contradict the TGA, without providing any of the evidence that TGA says is missing, in support of its position?
Update: The Philippines National Institute of Health recommended against the use of the Novavax in pregnant, breastfeeding and immunocompromised individuals, citing the lack of evidence of efficacy in these groups (since they were excluded from clinical trials) and the likelihood of an unfavourable risk:benefit ratio:
“In the case of populations who were at high risk of severe infection and are immunocompromised such as the HIV positive individuals and in the pregnant and lactating women, the Panel took into consideration the lower efficacy estimates and the wide confidence interval with the lower border breaching the 30% threshold combined with the signal of additional harm with the higher adverse reaction risk so that a recommendation against the use of the vaccine was given.”
Is NVX-Cov2373 (Novavax) effective and safe in the prevention of COVID-19 infection?
Doesn’t it warm your heart when a developing nation places more weight on the safety of its most vulnerable citizens than a rich nation like Australia?
Adverse event reports call for caution
Nuvaxovid was only granted provisional approval in Australia in late January 2022, but adverse event reports are already piling up.
AuxVaxSafety, which solicits adverse event reports from a subset of vaccine recipients on the third day after vaccination, reports that 2 in 100 Australians who received Nuvaxovid have sought their doctor’s advice or presented to an emergency department in the days after their first dose, and 15% were unable to attend work or school or perform normal duties.
As of 18 March 2022, 233 adverse reactions to Nuvaxovid had been reported to TGA’s Database of Adverse Event Notifications (DAEN), including 61 cases of chest pain, 29 cases of chest discomfort, 8 of pericarditis and 6 of hypertension, along with 60 cases of headache, and 56 cases of paraesthesia (a sensation of pricking, tingling, or creeping on the skin having no objective cause and usually associated with injury or irritation of a sensory nerve or nerve root).
There are some rather graphic descriptions of three of these adverse reactions, drawn from social media posts made by the people who suffered them, here.
Even if adverse reactions to the Novavax vaccine are rarer than to the AstraZeneca, Pfizer and Moderna shots, any risk at all is, in my opinion, a completely unacceptable price to pay for a product that offers no clinically meaningful benefit to individuals nor any social benefit.
So, don’t bother inviting me to join your Novastan cult. I’m staying in the control group.
For information on my private practice, please visit Empower Total Health. I am a Certified Lifestyle Medicine Practitioner, with an ND, GDCouns, BHSc(Hons) and Fellowship of the Australasian Society of Lifestyle Medicine.
Unlike the mRNA and viral vector injections, Novavax’s product does fulfil the technical definition of a vaccine as a preparation containing either a live, dead or attenuated pathogen or toxin.












Just another saponin adjuvanted killer shot like this one:
SHINGRIX vaccine is unsafe and its approval must be revoked
https://doi.org/10.5281/zenodo.1038301
Robyn superbly written as usual. I have been lazily waiting for someone to explain "why" this vax is any different. Talk about spin...they had us non mrna spike vaxers believing this one may be ok....whoa...just as bad . Wont be getting this either. Curiously though... I think i may have had it. Took about 5 days start to finish...high fever with hot n cold body aches, mild headache, fairly fatigued but worst was a severe gut pain...which when "passed through" ended the illness...or was it what I thought...a bad case of giardia/crypto...im fine now but how would I know. ?? Lol. Not going to any docs. Anyways novax on novavax!!