The DMSO Files: Part 2
Choose Your Conspiracy Theory
I’ve spent much of the last month deep diving into the published research on dimethyl sulphoxide (DMSO), testimonials and case series enthusing over its miraculous healing effects, pro-DMSO books and articles, anti-DMSO articles, articles that attempt to take a balanced viewpoint... and here’s what I’ve concluded: the DMSO story is a conspiracy theorist’s wet dream.

So many conspiracy theories; so little time
The leading conspiracy theory is that the US Food and Drug Administration (FDA) waged a ruthless war on DMSO despite promising early research, glowing reports from doctors and a groundswell of public enthusiasm for the substance, precisely because it is so effective at healing a stupendously wide array of diseases and conditions that it threatens the business model of Big Pharma, to which it is beholden. This conspiracy theory fails to adequately explain the fact that the biggest of Big Pharma corporations were falling all over themselves to file New Drug Applications (NDAs) for DMSO with the FDA in the early 1960s, but the FDA knocked them back. If the FDA only exists to serve the interests of drug companies, why didn’t it serve them when they wanted to develop DMSO as a drug?
Countering this is the pro-orthodox medicine conspiracy theory, which posits that DMSO is just a very useful solvent, which is cynically touted as a cure-all by quacks who shamelessly exploit the gullible public’s bottomless appetite for conspiracy theories that Big Government and Big Pharma are colluding to deny them effective remedies for their ailments.
Then there’s the anti-orthodox medicine and anti-‘alternative medicine’ conspiracy theory that Crown Zellerbach, the paper making company that provided Dr Stanley Jacob with his initial supply of DMSO (as discussed in Part 1), hyped this derivative of dimethyl sulphide (a byproduct of its wood pulping process) so that it could profit from two patents on the medicinal use of DMSO that it co-filed with Jacobs, rather than continuing to pay to dispose of its industrial wastes. And, as an added bonus, the promotion of DMSO would keep the public hooked in to relying on pharmaceuticals, rather than learning how to heal themselves. This conspiracy theory is somewhat undermined by the fact that Crown Zellerbach officials publicly announced that DMSO sales “would have little effect” on the company’s bottom line, which is not what one would expect if they were attempting to talk up the product’s financial prospects.
A more eyebrow-raising conspiracy theory posits that DMSO use is being heavily promoted by various assets of the predator class (including leading ‘alternative’ practitioners), because connecting people into the Internet of Things requires that the human body be made more permeable, a task for which the ‘universal solvent’ is uniquely suited.
No doubt, there are other conspiracy theories swirling around DMSO. But here’s one that came to my mind as I delved into the history of DMSO’s discovery, its exploration as a drug candidate, and the tussles between its promoters and the FDA: The media organs of the American establishment were absolutely pivotal in bringing DMSO to widespread public attention. And one of those media properties - CBS - intentionally influenced a Congressional hearing on its use. Just why they did this, was not explained by any of the sources that I read. But, being familiar with Operation Mockingbird, my conspiracy spidey-senses began to tingle when I read the list of publications that fermented DMSO fever, in two (seemingly) coordinated waves: the first in the early to mid 1960s, and the second in the early 1980s.
As mentioned in Part 1, The Oregonian newspaper ran an article featuring anecdotes about Dr Stanley Jacob’s successes in treating various minor ailments with DMSO, in December 1963. It’s just the kind of story that ticks all the boxes for the editor of a local paper: local doctor treats local people’s aches and pains with a handy-dandy new drug produced from a byproduct of a local industry.
But just one week later, the New York Times ran a story on DMSO, talking up its “anti-inflammatory and pain-killing properties” and describing results gained from its use as “unbelievable”. The article quoted “A physician at the medical school - not Dr. Jacob - [who] said yesterday that he had used it on a dozen patients, ‘and it is the most exciting thing I’ve seen in medicine.’” A similarly breathless story on the discovery of the new wonder-drug appeared five days later in Newsweek. US News and World Report, Life and the Saturday Evening Post followed in quick succession.
In late 1965, the FDA pulled the plug on NDAs for DMSO and prohibited all human testing due to concerns about eye damage reported in Merck’s application, and the death of a woman in Ireland “after three days of DMSO treatments for a sprained wrist”. By this time, an army of DMSO advocates had formed - not least due to the heavy promotion of DMSO by the most influential publications in the country - and this pro-DMSO movement proceeded to deluge the FDA for the next 20 years with demands to cease its persecution of the wonder-drug that many credited with transforming, and even saving, their lives.
The second wave of media-generated hype around DMSO began in March 1980, when CBS aired a story called “The Riddle of DMSO” in its flagship Sixty Minutes program. The program featured sympathetic interviews conducted by CBS’ Mike Wallace with Dr Stanley Jacob and some enthusiastic users of DMSO, along with footage of Wallace - in his trademark aggressive style - confronting an FDA official with several journal articles attesting to DMSO’s benefits. CBS had intentionally timed the program to air the night before a scheduled hearing of the House Select Committee on Aging, on DMSO and arthritis. The unabashedly pro-DMSO Sixty Minutes program - and the reaction to it by many of the estimated 37 million Americans who watched it - influenced several congressmen to introduce bills requiring FDA to authorise studies on the use of DMSO for scleroderma, brain injury, and arthritis.
Popular magazines and newspapers followed up the Sixty Minutes program with a deluge of articles extolling the miraculous benefits of DMSO. Between 1980 and 1981, Better Homes and Gardens, Fate, McCalls, Newsweek, People, Runner’s World, Sports Illustrated, Time, Vogue, and the Wall Street Journal all published pieces focusing on the reports of patients and doctors who swore by the healing powers of DMSO.
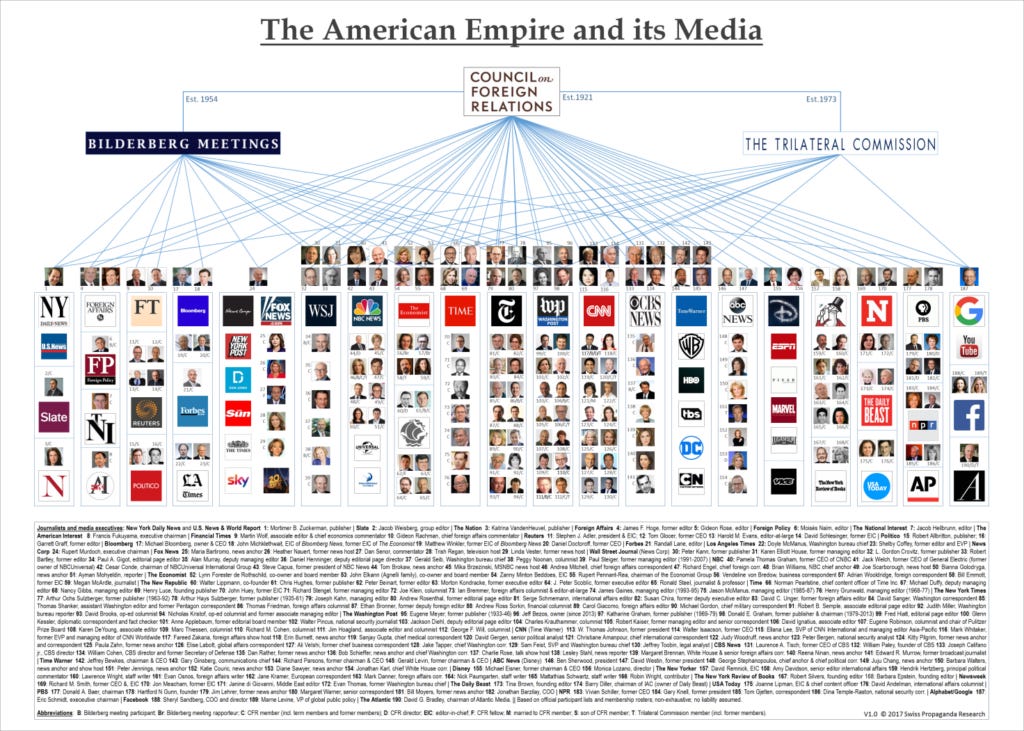
So what, you might be thinking. Just media hopping on a bandwagon, as they so often do. But if you have ever perused this diagram...
... or read about Operation Mockingbird - the CIA’s program to influence American media outlets and thereby, public attitudes - you might be wondering, as I did, whether there’s a little more to it than that. The CIA acknowledged New York Times, CBS and Time Inc (publishers of Time and Life magazines) as their three most valuable Mockingbird associates, with the Saturday Evening Post also an acknowledged participant.
And when the Democratic National Committee (DNC) wanted to squelch public interest in the alleged involvement of Bill and Hillary Clinton in the arkancide ‘suicide’ of Vince Foster, they paid $150 000 to Mike Wallace to do a Sixty Minutes hatchet job on a reporter who had gathered extensive evidence that the official story of Foster’s death was bunk. Allegedly.
By the way, here’s Mike Wallace at an exclusive party on the Lady Ghislaine superyacht, hobnobbing with notorious serial corporate criminal and Israeli intelligence asset Robert Maxwell - father of Ghislaine Maxwell, Jeffrey Epstein’s partner in underage sex trafficking - and Donald J. Trump:

Now, is it possible that Mike Wallace could be a paid propagandist for the political establishment, best buds with major financial criminals, and have a profound and genuine concern for regular American folks who just wanted to get some DMSO to ease their arthritic joints? I guess stranger things have happened. But I’ve acquired a deep cynicism for the regime media and anyone who works for them. When it comes to journalists within lamestream media outlets, I assume until proven otherwise that if their lips are moving, they’re lying.
What might be the purpose of a coordinated media campaign to push DMSO into public awareness? Two possibilities immediately spring to mind. First, and most obviously, amping up public interest in a product that several pharma giants are competing to commercialise (as was the case for DMSO in 1963, when the first wave of pro-DMSO articles were published), creates opportunities for financial speculators. Secondly, promoting any substance as a wonder-drug is actually a form of mass social conditioning which primes people to believe that health is attained by taking in something from outside oneself, rather than by correcting the causes of disease and supplying the preconditions of health. A population that believes in wonder drugs is rendered permanently dependent.
Shifting out of speculation mode, let’s return to the nub of the dominant DMSO conspiracy theory: the claim that the FDA has spent decades suppressing DMSO because it just works too dang well to let the public have access to it.
The DMSO goldrush
Buoyed by Stanley Jacobs’ initial findings on the apparent safety and broad array of medical applications for DMSO, the Crown Zellerbach Company submitted preliminary data affirming its low toxicity to animals to the FDA, and subsequently received a Notice of a Claimed Investigational Exemption for a New Drug (IND), which permitted restricted tests on humans. More than 30 pharmaceutical companies competed to win research licences from Crown, which would permit them to conduct safety and efficacy testing on humans with the goal of submitting a New Drug Application (NDA) to the FDA.
Crown selected six of the largest outfits: Merck and Company, Geigy Pharmaceuticals, American Home Products Corporation, Syntex Corporation, the U.S. branch of the West Germany-based Schering Corporation, and Squibb and Sons. Over the next year, these companies invested in research on developing DSMO for treatment of various conditions. Three of them - Merck, Squibb, and Syntex - submitted NDAs to the FDA in early 1965, while Schering received approval to market DMSO in Germany in August of that year.
DMSO was on track to become a blockbuster drug... until a series of events occurred in late 1965, that completely derailed it. Firstly, FDA officials became concerned about data included in Merck’s NDA, which reported abnormal changes in the lenses of the eyes of laboratory animals treated with DMSO. And then, in September 1965, a woman in Ireland died of a suspected allergic reaction, after using DMSO on her sprained wrist for three days. Two weeks after the woman’s death was reported, FDA denied the three NDAs filed by Merck, Squibb, and Syntex, and yanked Crown’s IND. In November 1965, the Huntingdon Research Centre in Great Britain reported DMSO-induced eye damage to laboratory dogs, rabbits, and pigs; German drug companies voluntarily ceased marketing their DMSO products; and finally, the FDA Commissioner vetoed all further tests of DMSO on humans.
It’s impossible to understand the FDA’s decision-making without grasping the impact that the thalidomide tragedy had on drug regulators. Prior to 1962, the FDA had limited powers to prevent the sale of drugs. Pharmaceutical companies only had to meet fairly basic safety criteria, and no efficacy criteria, in order to market their wares in the US. Evidence emerged in late 1961 that thalidomide, a sedative and anti-nausea drug which was heavily promoted to pregnant women in 46 countries including Australia, New Zealand, Canada and several European countries, had caused thousands of miscarriages, and horrendous birth defects in more than 10,000 children.
Notably, thalidomide was never approved in the US, thanks to a single FDA drug reviewer, pharmacologist Frances Oldham Kelsey.1 Kelsey repeatedly delayed approval for the drug, insisting that more safety data were required. The sobering realisation that the US had so narrowly dodged the thalidomide bullet led to the passage of the Kefauver-Harris Drug Amendment of 1962, which required “substantial evidence” of both the safety and efficacy of a new drug, before it could be approved.
“‘Substantial evidence’ was defined as ‘evidence consisting of adequate and well-controlled investigations, including clinical investigations by experts qualified by scientific training and experience to evaluate effectiveness of the drug involved’ (Congressional Quarterly Almanac, 1962:199).”
An Incipient “Wonder Drug” Movement: DMSO and the Food and Drug Administration, p. 199
In the shadow of the thalidomide catastrophe, FDA officials were now setting a high bar for drug safety, and it didn’t take much for DMSO to stumble at that bar.
Enormous pressure was almost immediately brought to bear on the FDA to reverse its ban on testing DMSO. Members of the public wrote letters and signed petitions; doctors and medical researchers insisted that that there was no evidence of eye damage in humans; and a handful of congressmen decried the FDA for “persecuting” DMSO. In December 1966, the FDA relented, permitting trials of external application of DMSO in patients with conditions for which there was no existing effective treatment, with the proviso that test subjects must receive eye examinations every three months.
Squibb accepted the terms, testing DMSO on 127 prison inmates for up to two months (notably, falling short of the three-month threshold for eye examinations). They reported no evidence of eye damage, which then led the FDA to approve the testing of DMSO for up to two weeks for treatment of sprains, bursitis and other soft tissue injuries.
Under relentless pressure from the pro-DMSO movement to further loosen its restrictions on human testing, in 1971 the FDA commissioned the National Academy of Sciences (NAS) to review the drug’s safety and efficacy.
“The committee concluded that there was inadequate evidence of effectiveness in any disease and that the toxicity potential was sufficient to warrant DMSO remaining an investigational drug.”
An Incipient “Wonder Drug” Movement: DMSO and the Food and Drug Administration, p. 201
Then, in 1974, the FDA approved two studies, supervised by Stanley Jacob, to test DMSO’s effectiveness for interstitial cystitis - a painful, non-infectious bladder condition - and scleroderma, a disorder of collagen synthesis which leads to painful thickening and hardening of the skin and other organs. During the scleroderma trial, four of the 11 participants developed progressive myopia (shortsightedness) - the first evidence that DMSO adversely affected human eyesight. The FDA eventually rejected the NDA for scleroderma in 1978, on the grounds that the study lacked an adequate control condition, but approved the application for instillation into the bladder, for interstitial cystitis.
Subsequently, FDA Commissioner Jere Goyan, a pharmacist, personally reviewed the research conducted for the scleroderma NDA. Alarmed at its poor quality, he called for re-examination of the interstitial cystitis research. That review revealed that Stanley Jacob had written personal cheques totalling $36,500 to Dr Kaniambakam Chakra Pani, the FDA medical officer charged with reviewing all DMSO NDAs, including the one for interstitial cystitis that had eventually been approved. Jacob admitted that he had made the payments, but denied that he had done so in order to influence Pani to approve the NDAs, instead claiming that he had loaned Pani the money to help him pay his wife’s medical bills.
Jacob and Pani were charged with conspiracy, and with (respectively) giving and receiving an illegal gratuity to a federal officer. A hung jury resulted in the judge declaring a mistrial; a second attempt at prosecuting Stanley was eventually abandoned, because prosecutors feared that his immense public popularity - and widespread public mistrust of the FDA - would cause the jury to acquit him. Pani retired from public life, but Stanley’s brush with the law did nothing to dampen the ardency of DMSO true believers’ support for him.
Safe and effective - according to whom?
To this day, interstitial cystitis remains the only disease for which DMSO is an FDA-approved treatment, despite claims for its efficacy for a broad swath of acute and chronic conditions.
It’s important to bear in mind that the FDA itself does not conduct any testing of drugs; instead, it establishes criteria that sponsors of new drugs must meet during their testing process. Both medical and lay advocates of DMSO criticise the FDA’s criteria for establishing DMSO’s safety and efficacy. The former argue that the existing scientific literature already attests that DMSO is both safe and effective; the latter insist that their subjective experience constitutes sufficient evidence that the drug is safe and effective, and hence should be made freely available to the public.
DMSO advocates insist that the FDA’s requirement for double-blind randomised placebo-controlled trials to establish efficacy is inapplicable to a substance that causes a distinctive garlic breath and garlicky skin odour in most users, that causes both participants and doctors to break blind. (Some researchers have side-stepped this problem by using a 5 per cent solution of DMSO as the control condition - a high enough concentration to cause garlic breath, but - by most accounts - too low to achieve therapeutic benefit.)
Stanley Jacob believed that the FDA was too constrained - both intellectually and from a regulatory perspective - to deal with DMSO as a “therapeutic principle” rather than a drug. Jacob argued that the FDA’s interpretation of the Kefauver-Harris Amendment led it to consider that each drug could be approved for only one indication; faced with multiple NDAs for a plethora of applications for DMSO, it reflexively rejected them all until, eventually, grudgingly approving the application for interstitial cystitis.
At the end of the day, I don’t find the leading pro-DMSO conspiracy theory - the claim that the FDA has doggedly blocked and suppressed DMSO because it’s so astonishingly effective at treating a broad range of diseases, that it would threaten the dominant pharmaceutical model - terribly persuasive. The FDA’s decision-making has to be considered in its historical context, both with respect to the thalidomide tragedy and the regulatory changes that flowed from it, and evolving definitions of “substantial evidence” of efficacy.
I find it somewhat ironic that the same people who (quite rightly) castigate the FDA for cavalierly promoting the experimental COVID gene therapy shots masquerading as vaccines as “safe and effective” when they were no such thing, criticise the FDA for refusing to anoint DMSO as a “safe and effective” treatment for a wide range of conditions for which we have only anecdotal evidence, or poor-quality studies.
Coming up next...
Speaking of that wide range of conditions, I’ll be turning my attention to the quality of evidence for the use of DMSO for specific health problems including arthritis, interstitial cystitis, stroke and other neurological conditions, in the next few posts. I know this is what many of my readers have been anxiously waiting for, so thank you for your patience as I’ve spent the last few posts meandering down the historical and philosophical side-branches of the DMSO story! I hope that I’ve provided you with some important context that will help you make sense of the tangled web of claims and counterclaims about this contentious substance.
And if you want to ditch Substack and get my posts straight from the source - as well as commenting and viewing any content that Substack won’t let you see without using their privacy-invading facial recognition app - just
For information on my private practice, please visit Empower Total Health. I am a Certified Lifestyle Medicine Practitioner, with an ND, GDCouns, BHSc(Hons) and Fellowship of the Australasian Society of Lifestyle Medicine.
A small number of thalidomide-associated birth defects did occur in the US, because of samples distributed to 1000 doctors as part of a clinical trial.


You're amazing.
Here's a conspiracy theory and a half, do you think there could be any truth to it?
https://emetatron.substack.com/p/excerpt-from-soon-to-be-published?utm_source=post-banner&utm_medium=web&utm_campaign=posts-open-in-app&triedRedirect=true
You might want to connect to A Midwestern Doctor here on Substack as he has acquired a large number of the studies done on DMSO. I personally have been using on myself and pets for a variety of things with great results.