Which foods boost GLP-1?
Are there foods, or dietary patterns, that assist weight loss by naturally boosting GLP-1? You betcha!
Back in Part 1 of this mini-series on GLP-1 agonists, I explained that these drugs are incretin mimetics. That is, they mimic the effects of biochemicals - including glucagon-like peptide 1 (GLP-1) - that potentiate the release of insulin after meals.
You might also remember that GLP-1 has a half-life of less than two minutes, whereas the GLP-1 agonist drugs have much longer half-lives: 13 hours, in the case of liraglutide, and for semaglutide, 7 days. As I discussed in Part 2, many of the side-effects of GLP-1 agonists, especially the gastrointestinal ones such as nausea, vomiting, constipation, diarrhoea and gastroparesis, are attributable to this extreme prolongation of the normal duration of activity of naturally-produced GLP-1.
Whilst pondering the myopia and hubris of the pHarma researchers who somehow think it's a good idea to monkeywrench human physiology, I suddenly remembered the Sorcerer's Apprentice segment from the (pre-woke) Disney classic, Fantasia. In this scene, Mickey Mouse is the eponymous (or should that be eponymouse?) apprentice. Tasked with carrying heavy buckets of water from the well to fill the sorcerer's cauldron, Mickey opts to use magic instead. He casts a spell on a broomstick, which sprouts arms and takes over Mickey's chore. Puffed up with pride at his own proficiency, Mickey falls asleep and dreams that he is a mighty sorcerer, commanding the movements of the stars, planets and water. He wakes up to find that the room has flooded, as the spellbound broomstick has overfilled the cauldron, but will not cease carrying out its appointed task. All Mickey's desperate attempts to stop the broomstick from continuing to fill the cauldron only make the situation worse, until finally the sorcerer returns and breaks the spell. A chastened Mickey returns to carrying out his chores, with his own two arms and legs.
The difference between the Sorcerer's Apprentice segment and the unfolding GLP-1 agonist clusterf*#k is twofold: First, there is no sorcerer coming to save the day; and second, unlike Mickey, the scientists who develop these drugs never acknowledge their mistakes - mistakes that have resulted in horrendous side-effects and a growing number of deaths. Mickey recognises that his attempt to wield power beyond his control and comprehension led to disaster, and humbly returns to honest labour; drug developers see the failure of GLP-1 agonists to create sustainable weight loss as an opportunity to produce and sell even more drugs, as I discussed in Part 3.
But there is one valuable little gem hidden amongst the giant pile of GLP-1 agonist dross: GLP-1 is a potent biochemical with many beneficial effects on human health. In addition to its role in promoting insulin release, you might remember from Part 1 that GLP-1 also:
Slows down gastric emptying, the rate at which the stomach discharges its contents into the small intestine;
Increases satiety, the feeling of fullness after a meal which discourages further eating;
Decreases appetite centrally (i.e. within the brain), by enhancing the sense of satisfaction with the food you've already eaten, and decreasing the reward value of further consumption - especially of high-fat, high-calorie food;
Inhibits apoptosis ('cell suicide') of insulin-producing beta cells, thus preserving the capacity of the pancreas to produce insulin
Increases natriuresis and diuresis, the excretion of sodium and water via urine;
Decreases inflammation; and
Has protective effects on the heart (for example, improving coronary blood flow, cardiac output, and endothelial function) and nervous system.
To summarise, GLP-1 regulates blood glucose level, appetite and food intake by acting both on the organs of the digestive tract (especially the stomach and pancreas) and on the brain. Self-evidently, it has played a vital role in our species' survival and adaptation. After all, animals need some way of distinguishing when their energy reserves are running low, so that they will seek food, vs when their stores are replete and they can divert energy into other activities. GLP-1 is part of a complex network of signalling molecules that helps our brains to make wise resource allocation decisions.
Intriguingly, there is an inverse relationship between plasma GLP-1 levels and body mass index (BMI). That is, people who are overweight or obese have a lower GLP-1 response to food, than healthy-weight people. Most studies of type 2 diabetics and prediabetics have shown the same thing: a delayed and blunted rise in GLP-1 after eating, compared to nondiabetics. Their appetite-regulating mechanism is impaired, causing a vicious circle of overeating and weight gain.
This raises an interesting question: Do our dietary choices affect GLP-1 secretion and, thereby, impact appetite regulation and all the other actions exerted by GLP-1? And another question follows from that one: Might changes in the nature of the food supply have, via their effect on GLP-1, impacted appetite regulation and hence, contributed to the epidemic of obesity and diabetes?
The answers to those questions are yes, and hell, yes.
Let's set the table (pun intended) to understand the dietary modulation of GLP-1 secretion. You might recall from Part 1 that GLP-1 is released in a biphasic pattern. The first phase occurs within 10-15 minutes after food intake, and is believed to be triggered by neuroendocrine factors (i.e. hormones that are secreted in response to nervous system signalling that announces the arrival of food in the stomach). The second phase occurs 30 to 60 minutes after a meal, and is influenced by the arrival of macronutrients - carbohydrates, proteins and fats, or their constituent molecules - in the final segment of the small intestine (the ileum) and the colon.
The blood concentration of GLP-1 increases by two- to four-fold after a meal, reaching a peak about an hour after food ingestion and then gradually declining until the next eating occasion. But once again, you might recall from Part 1 that GLP-1 acts not only as a hormone (which must diffuse into the bloodstream to exert its activities) but also directly on sensory fibres of the vagus nerve, the 'ethernet cable' which connects the gut brain to the brain between your ears. In other words, GLP-1 released in the gut can directly signal, via the vagus nerve, to the appetite-regulating centres in the brain, sending the message that you've had enough to eat and should put that fork down now.
The density of the specific cells that secrete GLP-1 progressively increases, all the way down to the final segment of the colon. If you know anything about the human digestive tract, that should get your attention. Most digestion and nutrient absorption occurs in the small intestine. Yet there are more GLP-1-secreting cells in the colon, which was historically viewed as a waste-processing organ rather than as a nutrient-absorbing organ. That dismissive characterisation of the colon as merely a sewer has been turned on its head by research on the gut microbiome, which has exploded over the last couple of decades. It's now evident that the production and absorption of a particular group of nutrients, the short chain fatty acids, is one of the primary functions of the colon. More on this topic, shortly.
Hopefully, the pieces of the puzzle are starting to come together for you:
If the foods that you eat elicit more GLP-1 release, you'll feel full after eating fewer calories; AND
If those foods contain, and/or give rise to the production of, more of the macronutrients that trigger GLP-1 release, you will experience more prolonged GLP-1 signalling after a meal; AND
If those foods are also digested more slowly, you'll have even more prolonged GLP-1 signalling (and hence, appetite suppression) after you finish eating, and hence it will take longer before you feel hungry again; AND
If every meal that you eat is composed largely of foods that elicit higher, and more prolonged, GLP-1 secretion, you will spontaneously and effortlessly consume fewer calories than if you consistently eat foods that induce less GLP-1.
In combination, these four consequences of higher GLP-1 release make it easier for overweight people to put themselves into negative calorie balance (i.e. more calories burnt up than absorbed) for an extended period of time, and hence to lose weight and keep it off, without feeling hungry all the time.
Which specific macronutrients trigger GLP-1 secretion? Here's what's known so far:
Monosaccharides - that is, simple sugars, such as glucose and fructose. These are derived either from ingestion of foods rich in simple sugars (e.g. sugar-sweetened beverages and confectionery), or from the digestion of starches (e.g. potatoes, rice and bread). Remember, the density of GLP-1-secreting cells increases along the length of the intestines. Hence, the more slow-to-digest carbohydrate foods, such as sourdough rye bread or steel cut oats, will trigger higher, and more sustained, release of GLP-1 than sugary foods and rapidly-digesting starchy foods like white rice and white bread, whose monosaccharides are absorbed in the upper segment of the small intestine.
Monounsaturated and polyunsaturated fatty acids, especially unsaturated long-chain fatty acids. The majority of studies performed on humans have compared the effects of monounsaturated fat-rich olive oil to those of saturated fat-rich butter, and have found that olive oil consumption results in higher postprandial (after-meal) GLP-1 blood concentrations. Some studies also found improved insulin sensitivity, and decreased fasting and postprandial blood glucose concentrations, with olive oil.
Individual amino acids and short chains of amino acids, known as peptides, which are derived from the digestion of protein. Individual amino acids have been shown to stimulate GLP-1 secretion in vitro (i.e. in isolation, rather than in living organisms), and high protein meals lead to higher postprandial GLP-1 concentrations than low protein meals, but the specific mechanisms underlying protein-induced GLP-1 secretion remain poorly understood.
Short chain fatty acids including acetate, butyrate and propionate. These are derived from bacterial fermentation of non-digestible carbohydrates such as soluble fibre, oligosaccharides and pectin. This fermentation takes place in the colon, where the density of GLP-1-secreting cells is highest. The effects of fermentable fibres - found in plant foods such as fruits, many vegetables, legumes (dried peas, beans and lentils), whole grains (especially oats, wheat, rye and barley), nuts and seeds - on GLP-1 secretion, appetite, food intake and metabolism are quite remarkable:
"Compared to a standard control diet, the consumption of a diet enriched in fermentable fiber for 50 days increased GLP-1 concentrations in the proximal colon, which was associated with an increased expression of proglucagon [a precursor of GLP-1] in colonic L-cells [129]. Fermentable fiber also decreased food intake, weight gain, as well as blood triglyceride concentrations and triglyceride accumulation in the liver [129]... In healthy humans, a crossover pilot study showed that the [sic] daily supplementation with 16 g of fermentable fiber for a two-week period increased postprandial satiety, and decreased hunger, prospective food consumption as well as energy intake over 24 hours following the intake of a standard breakfast meal."
Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review
It is highly likely that the release of GLP-1 that is triggered by fibre in the colon, contributes significantly to the so-called 'second meal effect': if you eat a meal high in fermentable fibres, you'll be less hungry at, and have a better insulin response to, the next meal.
There is even tantalising evidence - only established in rats, at this point - that higher intake of fermentable fibres actually increases the number of GLP-1-releasing cells in the colon, creating a virtuous circle in which the more fibre you eat, the more meal satisfaction, satiety and prolonged appetite suppression you'll derive from high-fibre meals.
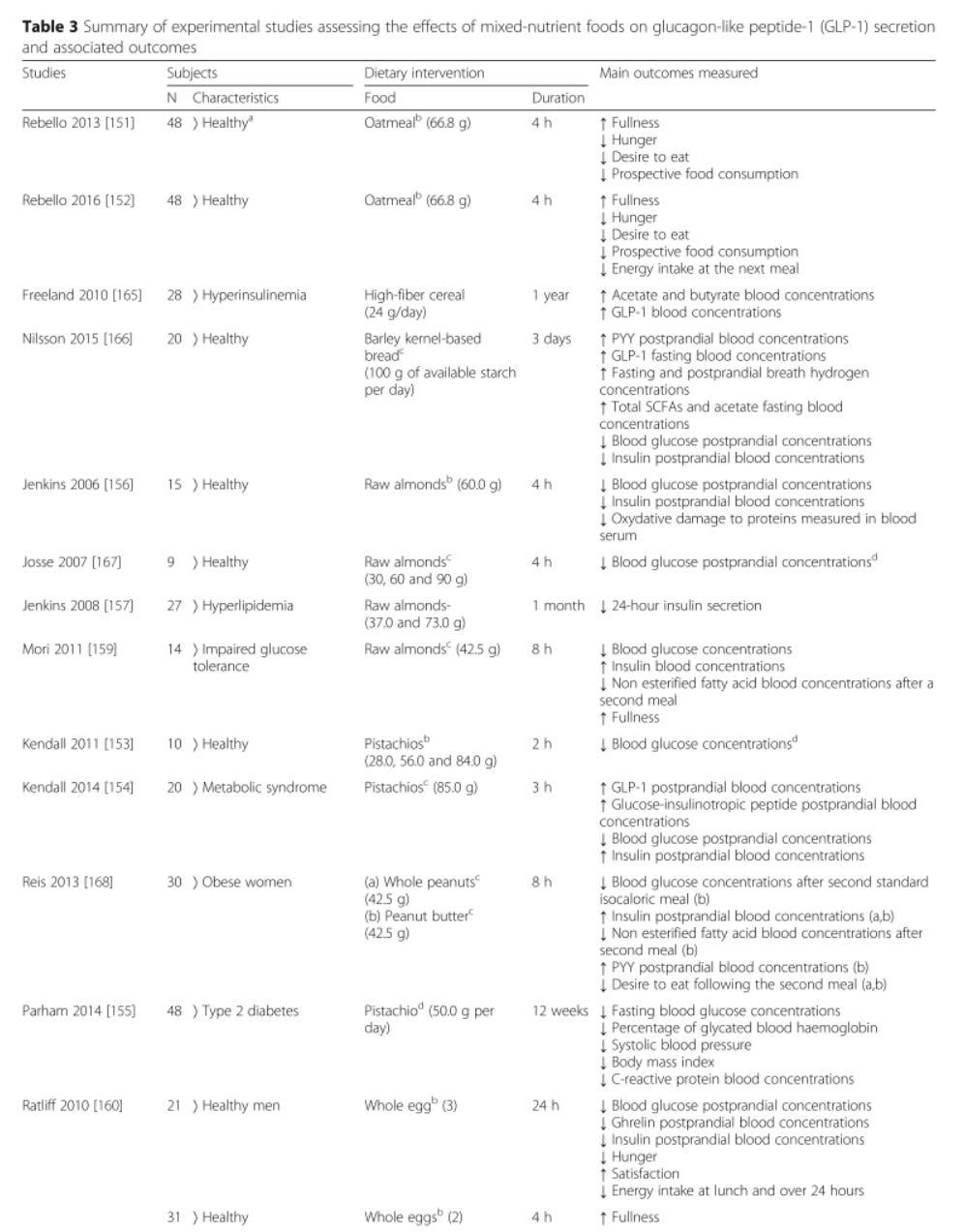
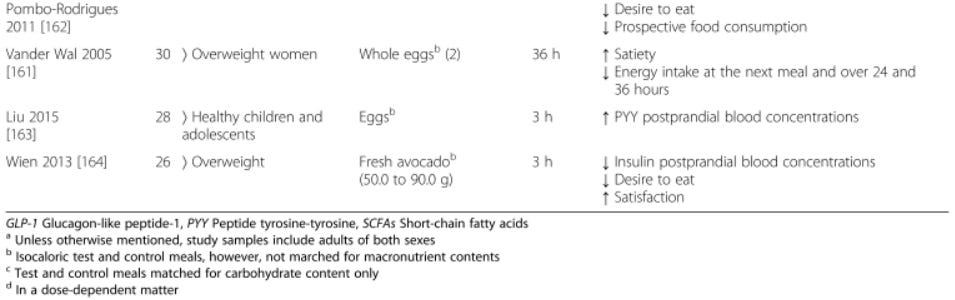
The table below is excerpted from 'Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review'; it summarises the effect of various dietary interventions on the secretion of GLP-1 and another appetite-suppressing hormone, peptide YY (PYY), as well as subjective and objective measures of hunger, fullness and subsequent food consumption:
Since that review was published, more studies examining the effects of particular foods and dietary patterns on GLP-1 secretion have come out:
In a randomised crossover study published in 2019, the incretin and insulin responses of 20 overweight or obese men with type 2 diabetes to a meat burger meal and a soy burger meal were studied. Each man served as his own control, eating the two burger meals in random order, with a one-week washout period in between. The meals were matched for energy and macronutrient content, but more of the carbohydrate in the meat-based meal came from sugar (rather than starch), and the meat-based meal had almost four times more saturated fat and less than a third as much fibre as the soy-based meal. Plasma concentrations of GLP-1 were almost 20 per cent higher after eating the soy-based burger, and several markers of beta cell function were also improved. Satiety and hunger perceptions were not assessed in this study.
In a randomised crossover study of a high-fibre vegetarian meal vs a Mediterranean meal which was higher in protein, total fat and monounsaturated fat but lower in carbohydrate and fibre, the Mediterranean meal elicited a similar initial increase in GLP‐1 secretion to the vegetarian meal, but GLP-1 levels remained higher for longer after the Mediterranean meal. Interestingly though, there was no significant difference in ratings of fullness and desire to eat between the two meal conditions although, if anything, participants reported less hunger and desire to eat after the high-fibre vegetarian meal.
Another randomised cross-over trial compared the acute effects on appetite and gut hormones of two plant-rich Palaeolithic-type meals to a control meal based on World Health Organisation recommendations. The three test meals had similar amounts of carbohydrate and total fat, but both the Palaeolithic-style meals had more fibre, and one had more protein, than the control meal. Both the Palaeolithic-style meals induced higher and more prolonged GLP-1 secretion than the control meal, with the high protein meal eliciting a strikingly greater GLP-1 response. Satiety scores were higher after both Palaeolithic-style meals, but intriguingly, the lower-protein Palaeolithic-style meal evoked the greatest level of satiety per kJ despite eliciting less GLP-1 secretion.
Middle-aged, overweight and obese Iranian women who were randomised to follow a calorie-restricted MIND diet, which emphasises consumption of green leafy vegetables, berries, legumes, olive oil, fish, and chicken, with reduced intake of butter, cheese, red meat, fast food, and sweets, had higher fasting GLP-1 after three months, while women assigned to a calorie-restricted control diet saw their GLP-1 decline.
In in vitro studies, monosodium glutamate (MSG) reduced GLP-1 secretion after prolonged exposure (72 hours). MSG is widely used as a flavour enhancer in processed foods; the average intake of MSG is around 0.4 g per day in Europe, but excessive consumers in the UK have been reported to take in 4.68 g per day - more than ten times the European average.
And finally, good news for chocolate lovers: A cacao polyphenol-rich chocolate increased plasma GLP-1 and improved glucose metabolism in healthy, normal-weight Japanese adults, during a 50 g oral glucose tolerance test.
What does it all mean?
Regulation of human appetite, food intake and body weight and composition are complex and multifactorial. There is no 'magic bullet' solution to these complex problems, whether it be GLP-1 agonist drugs, eliminating "seed oils" or lectins or gluten from one's diet, or any other single manoeuvre touted by snake oil salesmen of various stripes.
But the lessons learned from studying the impacts of dietary components, and dietary patterns, on GLP-1 secretion, hunger and appetite can be synthesised into a guiding principle which knits together themes that I explored in Diving down the low-carb rabbit hole – Part 1 and Part 2, Ultra-processed foods: Cheap, tasty, convenient, and deadly, and the GLP-1 agonist mini-series:
Dietary patterns based on whole and minimally processed foods, with a high proportion of plant foods, facilitate attainment and maintenance of healthy body weight, metabolism and body composition, through a multitude of mechanisms.
Among these mechanisms is that such dietary patterns cause more GLP-1 release and greater subjective feelings of satiation and fullness. And, as the authors of the review article on nutritional modulation of GLP-1 secretion point out,
"In comparison with the use of GLP-1Rs agonists and DPP-IV inhibitors, targeting endogenous pathways through dietary modifications has the added benefits of potentially stimulating the release of other anorexigenic [appetite-suppressing] gut peptides (e.g. PYY), promoting beneficial changes in several markers of cardiometabolic health such as helping normalize blood lipid profile and blood pressure, as well as avoiding side effects associated with medication."
Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review
By contrast, processed foods - and most particularly, ultra-processed foods - are not only very energy dense, but also too rapidly and easily digested, and thus they do not elicit satiation (fullness after appropriate energy intake) or satiety for prolonged periods. People who eat ultra-processed foods on any more than an occasional basis are inevitably driven to overeat. And, sooner or later, depending on their age, sex, and lifestyle and genetic factors, they will gain weight.
In fact, as I discussed in a previous article, Ultraprocessed foods make you overeat – even when you don’t want to – and cause weight gain, in a landmark study published in 2019, Kevin Hall demonstrated that just two weeks on an ad libitum (eat as much as you want) diet of ultra-processed foods caused healthy young adults to gain an average of 900 g. On an unprocessed diet comprised mostly of fruit, vegetables, whole grains, legumes and nuts, with small amounts of meat and plain dairy products, the same young adults inadvertently consumed 508 fewer kCal per day, even though they were instructed to eat as much as they desired. As a result, they lost 900 g after two weeks.
I know that many people reading this article will be rolling their eyes and sighing "Duh! Tell us something we don't already know!" But others will be feeling disappointed that there's no secret GLP-1-activating weight loss plan built on expensive superfoods from exotic locations. Well, there's not. The secret to weight loss is no secret at all: stop eating Calorie Rich and Processed (CRAP) food.
But if letting go of the idea that there's a magical solution to the obesity epidemic has left a bad taste in your mouth, perhaps you can at least savour this delicious irony: all over the world, teams of researchers are busying themselves designing and conducting intricate experiments that use high-tech methods to prove that human bodies thrive on simple, minimally-processed foods. Chew on that one.
This post has taken approximately 10 hours to research and write. If you feel you’ve obtained value from reading it, please consider a paid subscription:






For those not familiar with Fantasia (I recall seeing it on telly in the 1960s) and especially The Sorcerer's Apprentice, here is the latter on YouTube: https://www.youtube.com/watch?v=2DX2yVucz24.
I cited Mickey's frenzied efforts to halt the brooms, which only caused them to multiply: https://www.youtube.com/watch?v=2DX2yVucz24&t=340s in my article "Corrupted groupthunk ineptitude in virology may create further pandemics" https://nutritionmatters.substack.com/p/corrupted-groupthunk-ineptitude-in regarding gain of function research into viruses, now increased due to the COVID-19 pandemic which was caused by such dangerous research, leading to further pandemics.
No-one involved in this remarkable animation would still be alive. https://en.wikipedia.org/wiki/Fantasia_(1940_film)#The_Sorcerer's_Apprentice_2 Mickey Mouse's eyes were given pupils for the first time. Live actors in costume were filmed to guide the animators who created this lurid, almost phantasmogorical, accompaniment to Paul Dukas' 1897 "L'Apprenti sorcier": https://en.wikipedia.org/wiki/The_Sorcerer%27s_Apprentice_(Dukas).
I presume digestion is different in all of us. Eating mostly Mediterranean I should be slender.