Subclinical hypothyroidism
A sadly neglected cause of avoidable suffering, or an overtreated fad diagnosis?
As I mentioned in last week's post on iodine, I was prompted to delve into this topic by a comment made by Dr
, author of the Substack:It turns out that Jessica is far from being the only person wondering whether hypothyroidism is being overdiagnosed, and thyroid hormone replacement is being overprescribed. Much ink has been spilt on this topic in medical journals. More precisely, medical researchers and clinicians have been grappling with the question of what to do about subclinical hypothyroidism for decades.
As I'll explain shortly, the conclusion reached after weighing the evidence is that people with subclinical hypothyroidism should not be put on thyroid hormone replacement, unless they have certain other specific clinical features. But many doctors - especially those who bill themselves as 'functional medicine' or 'integrative' practitioners - routinely prescribe thyroid hormone replacement to patients with subclinical hypothyroidism. So yes, at least some of those 20-30 per cent of Jessica's patients who are on Synthroid (the most popular brand in the US of levothyroxine, the synthetic version of the principle thyroid hormone) are victims of overprescription.
Given that levothyroxine is now the most-prescribed drug in the US but the only valid indication for its prescription - overt hypothyroidism - occurs in less than ten per cent of the population, it's pretty clear there's a whole lotta overprescribing going on. And the costs of overprescription are not solely measured in dollars spent on unnecessary medication, but also in adverse events, and lost opportunities to correct underlying drivers of ill-health.
To get a firm grasp on all of this, we need to start with the basics of thyroid function and dysfunction.
Thyroid 101
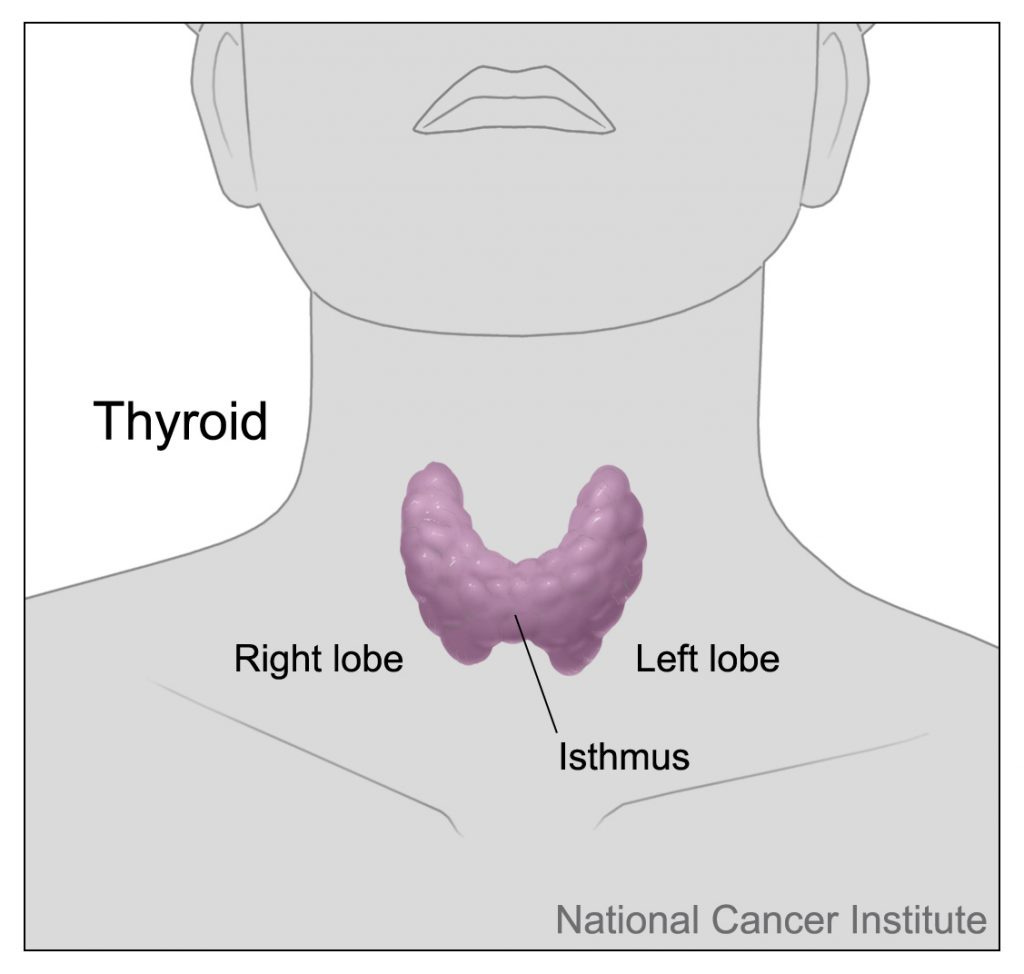
The thyroid gland (or simply, 'the thyroid') is a butterfly-shaped endocrine (hormone-producing) organ located at the front of the neck, below the Adam's apple. It comprises two lobes, connected by an isthmus:
The thyroid gland's job is, unsurprisingly, to make thyroid hormone. Or, to be more precise, to make thyroid hormones, of which the most frequently-discussed are thyroxine (T4) and triiodothionine (T3). (The red-headed stepchild of the thyroid hormone family is calcitonin, which helps to regulate serum calcium levels; I will perpetuate a long-standing tradition by completely ignoring it for the remainder of this article.)
Thyroid hormones have an astonishingly broad spectrum of activities on human physiology, which can be grouped into four categories:
Metabolic effects:
Increase basal metabolic rate by increasing mitochondrial activity, breathing rate, oxygen intake and consumption, which in turn leads to increased blood flow and core temperature.
Influence appetite, increase the breakdown of stored fat, protein and carbohydrate for energy.
Increase gut motility.
Increase cholesterol secretion in bile which leads to lower serum cholesterol.
Cardiovascular effects:
Increase heart rate and force of contraction.
Developmental effects:
Crucial for foetal brain maturation.
Increase growth rate in children and especially adolescents.
Stimulate bone maturation and growth.
Reproductive effects:
Influence libido.
Help maintain a normal menstrual cycle.
The regulation of thyroid hormone release is complex, involving a negative feedback loop in which two regions of the brain, the hypothalamus and pituitary gland, detect the level of thyroid activity and either nudge the thyroid to synthesise and release more thyroxine if there's insufficient levels, or less if there's too much. I really like this diagram, taken from an excellent video on thyroid hormones and thyroid function tests which you should watch if you want a thorough, yet concise, deep dive on this topic. The red arrows represent inhibitory pathways, and the blue arrows, stimulatory pathways.
The main hormones measured as part of thyroid function testing are thyroid stimulating hormone (TSH), which is released by the pituitary gland; thyroxine (T4), which is the main hormone released by the thyroid gland; and triiodothionine (T3), which is mostly formed in the tissues that respond to thyroid hormone signalling (pretty much every tissue in our bodies).
The intricate dance of thyroid hormone regulation can go tits-up in two broad ways: hypothyroidism - insufficient thyroid hormone production and/or activity - and hyperthyroidism - excessive thyroid hormone production and/or activity. As you might imagine from the broad suite of activities of thyroid hormone, both of these conditions produce a veritable Pandora's box of symptoms. Since this article was prompted by Jessica's question, I'm going to keep the focus on hypothyroidism. If you want me to delve into hyperthyroidism, let me know in the Comments section below, and I'll cover it in a future post.
Hypothyroidism
The textbook symptoms of thyroid underactivity are:
Fatigue
Intolerance to cold
Depression, slowed thinking, poor memory and concentration, diminished reflexes
Weight gain/difficulty losing weight despite reduced appetite
Heartburn, dysphagia, vomiting, dyspepsia, intestinal motility disorder, bloating and constipation
Non-restorative sleep
Slower heart rate, elevated blood pressure and cholesterol
Hoarse or husky voice
Fluid accumulation
Hair loss (especially the outer third of the eyebrows); thin, brittle, dry hair
In females: heavier and more frequent periods, anovular cycles.
The many faces of hypothyroidism
Hypothyroidism can be either primary - due to inability of the thyroid gland to produce sufficient hormones - or secondary - due to dysfunction of the hypothalamus or pituitary gland. Secondary hypothyroidism is quite rare, so I'm focusing this article on primary hypothyroidism.
There are two major groups of aetiologies (causes) of primary hypothyroidism: those caused by autoimmunity (i.e. the immune system producing antibodies that attack the thyroid gland) and those not related to autoimmunity.
Hashimoto's thyroiditis (also known as chronic autoimmune thyroiditis and chronic lymphocytic thyroiditis) is the most common cause of hypothyroidism in developed countries with generally iodine-replete populations. Postpartum thyroiditis is another relatively common autoimmune thyroid disease, which causes transient hypothyroidism in between two and ten per cent of women in the first year after giving birth.
The non-autoimmune causes of hypothyroidism can be grouped into exogenous - drugs, surgery, radiation - and endogenous - relating to iodine intake, which I'll discuss at some length below.
Yet another way of classifying hypothyroidism is overt vs subclinical.
As the video linked above explains, people with overt, or clinical, primary hypothyroidism, have an elevated TSH with low T4 and/or T3. Recalling the negative feedback mechanism depicted in the diagram above, essentially what is going on here is that the pituitary gland is trying to kick the thyroid into producing more T4, but the thyroid is too clapped-out to respond. If these findings persists on a subsequent blood test, the patient will be prescribed levothyroxine (synthetic thyroxine); or, much more rarely, levothyroxine plus liothyronine, a synthetic triiodothyronine; or more rarely still, desiccated thyroid glands derived from animals. Patients are almost always told that they will need to take thyroid hormone replacement for the rest of their lives.
In subclinical hypothyroidism, the T4 and T3 levels are within the reference range, but TSH is elevated. 'Subclinical' means that the individual is not yet manifesting symptoms of hypothyroidism. However, many practitioners will tell patients that their vague and/or common symptoms such as fatigue, difficulty losing weight and brain fog are evidence of thyroid underfunction which requires treatment - that is, commencing Synthroid or some other form of thyroid hormone replacement.
But such symptoms are at least as common in people with no evidence of thyroid dysfunction as in those with subclinical hypothyroidism. For example, among attendees at a Colorado health fair, hypothyroid symptoms were reported by 12 per cent of individuals with overt hypothyroidism, 7.4 per cent of those with subclinical hypothyroidism, and 7.7 per cent of those who were euthyroid (no laboratory abnormalities in thyroid function). In other words, people with no detectable problems with their thyroids were about as likely to report experiencing some of the symptoms of hypothyroidism, as people with laboratory-identified subclinical hypothyroidism. And in a study of the residents of Tromsø, Norway, those identified as subclinically hypothyroid had no differences in either cognitive function or hypothyroid symptoms compared to euthyroid individuals, but had better emotional function.
And here's where Jessica's question comes into sharp focus. Given that only roughly 8 per cent of Americans aged over 12 have overt hypothyroidism, if 20-30 per cent of her patients are taking thyroid hormone replacement, then she either has somehow managed to attract a clientele whose rate of overt thyroid dysfunction wildly exceeds that of the general population, or, more likely, her patients are being overdiagnosed and overprescribed. The majority of people diagnosed with subclinical hypothyroidism never go on to develop overt hypothyroidism; the conversion rate is estimated to be 2 to 3 per cent per year of patients without thyroid autoantibodies, and 4 to 5 per cent per year when thyroid autoantibodies are present. A large proportion of the remainder spontaneously revert to normal thyroid function without any treatment at all: 60 per cent of people with elevated TSH are back to normal levels within 3 months, and 62 per cent after five years.
So is it appropriate to treat people who do not have overt hypothyroidism, with thyroid hormone replacement? For this to be a sensible course of action, the benefits of treatment would have to outweigh the risks. Specifically, if treating subclinically hypothyroid individuals with thyroid hormone replacement therapy is worth doing, it should reduce the rate of progression to overt hypothyroidism, and/or improve symptoms and lessen the risk of cardiovascular disease. AND it should do so without causing adverse effects that offset such benefits. The best way to determine the risk:benefit ratio would be a large randomised controlled trial with a lengthy follow-up period. Or even better, a meta-analysis of such trials. Et voilà:
"In this meta-analysis of 21 randomized clinical trials including 2192 participants with subclinical hypothyroidism, thyroid hormone therapy was not significantly associated with improvements in general quality of life (standardized mean difference, −0.11) or thyroid-related symptoms (standardized mean difference, 0.01).
Meaning
These findings do not support the routine use of thyroid hormone therapy in adults with subclinical hypothyroidism."
So treating people whose blood tests indicate subclinical hypothyroidism with levothyroxine doesn't make them feel any better overall, and it doesn't even relieve the specific symptoms associated with underactive thyroid. Only one of the included trials evaluated cardiovascular events or mortality, and found no benefit for either. (In fact, in older patients, subclinical hypothyroidism is associated with greater longevity. In participants of the Rotterdam Study who were aged over 50, men in the highest bracket of TSH lived two years longer than those with the lowest TSH; for women, the difference was 1.4 years.)
What's the downside of treating subclinical hypothyroidism?
Unlike your thyroid gland, which (when healthy) is able to precisely calibrate the amount of thyroid hormone that you need, and to adjust its output moment-by-moment, thyroid hormone replacement comes in fixed dosages. Overdosing is perilously easy, especially in people aged 65 and over, and can result in cardiac arrhythmia, progressive heart failure, increased bone turnover leading to osteoporosis, muscle wasting, impaired quality of life and increased mortality.
In a study of over 52 000 UK patients who were prescribed thyroid hormone replacement, after five years, 5.8 per cent had developed a suppressed TSH (<0.1 mIU/L) which is known to increase the risk of atrial fibrillation and osteoporotic fracture. The authors also noted that the median TSH level for initiating levothyroxine treatment dropped from 8.7 mIU/L in 2001 to 7.9 mIU/L in 2009, suggesting that doctors were prescribing thyroid hormone replacement at more marginal degrees of hypothyroidism.
Does anyone benefit from treatment of subclinical hypothyroidism?
Women with subclinical hypothyroidism (TSH > 4.0 mIU/L) who are planning pregnancy, or are identified during pregnancy, should commence treatment with levothyroxine to reduce the risk of miscarriage and pregnancy complications.
Subclinical hypothyroidism with TSH > 10 mIU/L warrants treatment, as this degree of TSH elevation increases the risk of heart failure and other cardiovascular events.
A type of heart failure known as left ventricular diastolic dysfunction appears to be linked to subclinical hypothyroidism, and is reversible with thyroid hormone replacement to normalise TSH.
And finally, if anti-TPO antibodies are present, thyroid hormone replacement therapy might be helpful. In my opinion though, other therapies for lowering thyroid autoantibodies, such as Nigella sativa seeds, myo-inositol, selenium and vitamin D, are worth trialling before resorting to levothyroxine or any other form of thyroid hormone replacement, because if you can arrest the destruction of the thyroid gland by autoimmune processes, it might recover its ability to produce an adequate amount of hormones.
The iodine connection
As mentioned in last week's post, iodine deficiency can cause primary hypothyroidism, simply because iodine is required for thyroid hormone synthesis. But paradoxically, excess iodine intake also causes hypothyroidism, and hypothyroidism is more prevalent in areas with excessive iodine intake than in iodine-deficient areas.
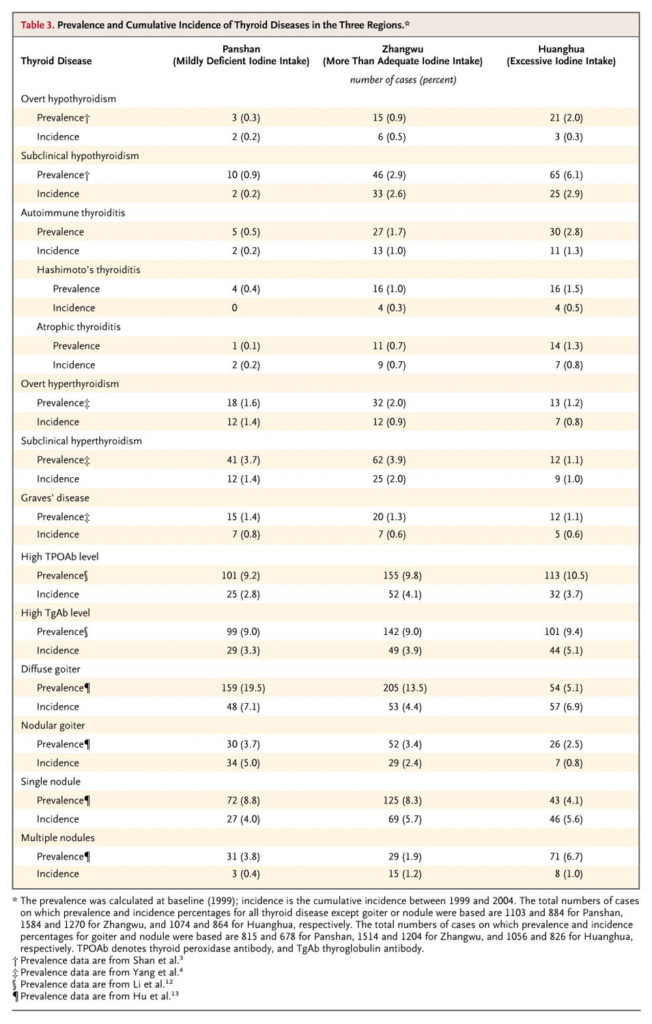
For example, a study conducted in China (which has had a nationwide salt iodisation program since 1996), compared rates of subclinical, overt and autoimmune hypothyroidism in regions with three different levels of iodine intake - slightly deficient, more than adequate and excessive. Iodine intake was assessed both by dietary questionnaire and by urinary iodine excretion (see the previous post). Both measures of iodine nutriture were assessed in 1999 and 2004, and found to be stable. Levels of TSH, T4, T3 and thyroid autoantibodies - which indicate autoimmune thyroid disease - were also measured. The lowest risk of each form of hypothyroidism was found in the mildly iodine deficient population. Autoimmune thyroiditis appeared to be the main cause of overt hypothyroidism:
A study of coastal Japanese people with varying levels of iodine intake, on the other hand, found that while hypothyroidism was both more prevalent and more severe in people who consumed the most iodine, there was only a correlation between high iodine intake and the non-autoimmune form of hypothyroidism.
What might account for these contradictory findings? The major sources of iodine in the Chinese study were iodised salt and drinking water, while seaweed provided most of the iodine in the Japanese study. Perhaps the nutritional matrix in which iodine enters the human body, influences its impact.
In any case, the link between higher-than-adequate iodine intake and hypothyroidism, documented in both these studies, was not a fluke. A systematic review and meta-analysis that included intervention trials, case-control studies, follow-up studies and cross-sectional studies, found that compared to adults with adequate iodine intake, those with excess intake (which came primarily from iodised salt or water) had 2.03 times the odds of subclinical hypothyroidism, and 2.78 times the odds of overt hypothyroidism.
Remember, iodine - like all other nutrients - has a Goldilocks zone, and because we require only microgram quantities of this essential nutrient, it's easy to tip over from adequate to excessive intake.
Conclusion
There's an unfortunate tendency to assume that 'getting a blood test' will help to identify the cause of common symptoms such as fatigue, difficulty in losing weight, and brain fog. More often than not, such scattershot testing identifies biochemical abnormalities which may have nothing to do with the presenting symptoms, and whose meaning is uncertain... but both doctor and patient often feel the need to do something about them.
Subclinical hypothyroidism is usually detected in such circumstances - or even as a result of 'routine' testing of patients with no symptoms at all. Unfortunately many people diagnosed with subclinical hypothyroidism end up taking thyroid hormone replacement therapy, which in most cases will do them more harm than good.
And there's an opportunity cost too, in that the focus on their newly discovered thyroid 'condition' directs attention away from addressing the fundamental diet and lifestyle factors that have the largest influence on health. If you eat a low-quality diet, poison yourself with alcohol or other drugs, don't get enough exercise or sleep, and are deprived of sunlight, loving relationships and a sense of purpose in your life, you're going to experience symptoms of ill-health that are sure as hell not going to be fixed by taking thyroid hormones (or anything else, for that matter). Health comes from healthful living, not from a packet of pills.
This post has taken approximately 20 hours to research and write. If you feel you’ve obtained value from reading it, please consider a paid subscription:







Thank you so much for the time it took to research and write this article. Very informative and so aligned with what I suspected. Since the symptoms of hypothyroidism are quite ubiquitous amongst many adults, it’s easy to see why medicine throws a pill at it to feel like something is being done. The harder work is do all the things you suggest, ie healthy living, sleeping, eating and loving.
You’re an amazing resource and we’re lucky to have you helping us to understand the complexity of our health.
Since moving to the Shoalhaven region in NSW in 2015 it has horrified me to meet so many women mostly between mid 30's to may be late 40's who have had their thyroid destroyed by radiation then forced onto Synthoid as a substitute for then rest of their lives. My sample is by no means representative and as an older male most women are not going to blab to me about their Thyroid condition. My blood boils though when I do hear this story as some medico's are making a fortune doing this to these women and I seriously doubt that it is either required or an effective treatment for whatever prompted the women to seek medical advice in the first place. There is a LOT of accounting to be done one way or another, someday and somewhere.