New hope for people with Alzheimer's disease
A ground-breaking new study provides compelling evidence that early-stage Alzheimer's disease is reversible
Dementia, including Alzheimer's disease, is now the second leading cause of death in Australia, and the leading cause of death for women. It is estimated that over 421 000 Australians are currently living with dementia. And that means, of course, that their spouses, children and grandchildren, other family members and friends are living with it too - living with the fact that their loved one is dying a death of a thousand cuts as their memories, personality and ability to carry out normal activities of daily living are relentlessly, remorselessly stolen from them, day by painful day.
As I discussed in my two-part series Preventing dementia, there's a wealth of research on the diet and lifestyle factors which help to prevent dementia. But what about reversing it once the process of brain degeneration is already underway? There are certainly no drugs that can do this:
"Despite the eye-popping sums poured into research on pharmaceuticals to treat Alzheimer’s – over US$42.5 billion of private funding between 1995 and 2021, and billions more in public funds – there are still no medical treatments that slow the progression of this devastating disease, or mitigate the underlying biological processes that cause it."
So the publication, just last week, of a paper titled 'Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: a randomized, controlled clinical trial', in the journal Alzheimer's Research & Therapy, has profound significance. The study reported in the paper found that:
"Comprehensive lifestyle changes may significantly improve cognition and function after 20 weeks in many patients with MCI [mild cognitive impairment] or early dementia due to AD [Alzheimer's disease]."
What exactly were these "comprehensive lifestyle changes"? The multimodal intervention program was as follows:
A whole foods, minimally processed plant-based (vegan) diet high in complex carbohydrates, and especially low in harmful fats (saturated and trans fats), sweeteners and refined carbohydrates. The diet comprised predominantly fruits, vegetables, whole grains, legumes, soy products, seeds and nuts. It provided approximately 14-18 per cent of total calories as fat, 16-18 per cent as protein, and 63-68 per cent as carbohydrates (mostly complex). To maximise compliance, participants received twice-weekly meal deliveries providing three meals and two snacks per day for themselves plus their study partner (spouse or co-resident), and were instructed to consume only the foods delivered to them for the duration of the study. Calories were unrestricted; extra food was delivered to people with higher calorie requirements.
The following nutritional supplements, which were delivered to participants with instructions to take all these, and only these, supplements each day throughout the 20 week study:
1680 mg omega-3 fatty acids with 800 mg curcumin
A multivitamin and mineral supplement without iron
200 mg coenzyme Q10
1 gram vitamin C
500 mcg vitamin B12
144 mg magnesium L-threonate
2 grams Hericium erinaceus (Lion’s Mane)
Multistrain Lactobacillus and Bifidobacteria probiotic
Exercise: Participants were instructed to engage in aerobic exercise such as walking for at least 30 minutes per day, as well as mild strength training exercises at least three times per week either with an exercise physiologist in person or via virtual sessions. Patients were issued a personalised exercise prescription based on age and fitness level.
Stress management: Participants were asked to do meditation, gentle yoga-based poses, stretching, progressive relaxation, breathing exercises, and imagery for a total of one hour per day, supervised by a certified stress management specialist. They were also given access to online meditations, and had the option of using flashing-light glasses at a theta frequency of 7.83 Hz plus soothing music as an aid to meditation and insomnia. Participants were also instructed to get adequate sleep.
Group support: Group support sessions, facilitated by a licensed mental health professional, were provided to participants and their study partners, to furnish emotional support and build community, as well as to develop communication skills and strategies for maintaining adherence to the program. These sessions also included memory exercises, which were detailed in a book provided to participants.
The level of support provided to participants and their study partners was quite breathtaking: Three times per week, a four hour session was conducted via Zoom, comprising one hour of supervised exercise (aerobic and strength training), one hour of stress management practices (stretching, breathing, meditation, imagery), one hour of support group and a one hour lecture on lifestyle; additional optional exercise and stress management classes were also provided for extra-keen participants.
The study group was headed up by lifestyle medicine pioneer Dr Dean Ornish, whose work on reversing atherosclerotic cardiovascular disease and early-stage prostate cancer I've discussed in previous articles. Ornish collaborated with an international team of neuroscientists, gerontologists, epidemiologists and other assorted specialists, including New Zealand's Rob Knight, a computational microbiologist and co-founder of the American Gut Project, a crowdsourced, global citizen science effort to map the human microbiome; and neurobiologist Rudolph Tanzi, Chair of the Cure Alzheimer’s Fund Research Leadership Group and discoverer of multiple genes associated with increased risk of Alzheimer's disease. In other words, not a bunch of rank amateurs, vegan ideologues or Instagram influencers.
Ornish's initial plan was to enrol 100 participants diagnosed with mild to moderate dementia due to Alzheimer's disease, but the recruitment process was hindered both by the COVID-19 policy response, and competition from pharmaceutical companies who were recruiting patients with similar criteria for drug trials. In the end, 51 patients were enrolled, with 26 randomised to the treatment group and 25 to the control group, who continued to receive usual care and were instructed to maintain their usual diet and exercise habits. (To deter the control group from making any lifestyle changes during the intervention period, which might have confounded the study results, they were all offered free participation in the multimodal program after the 20 weeks had elapsed.) Two patients assigned to the intervention group dropped out because they didn't want to continue with the diet and lifestyle changes.
All participants underwent a battery of clinical and cognitive assessments at baseline, and after completing the 20-week program. Stool samples were also collected at baseline and 20 weeks, to analyse gut microbial composition. The study protocol had initially included MRI and amyloid PET scans, but once again, the COVID-19 policy response foiled these plans, as many hospital facilities were shut down, awaiting the surge of COVID patients that never came (but freeing up nurses to polish their TikTok dance routines).
The four tests used to assess changes in cognition and function in participants were standard measures used in many FDA drug trials: Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog); Clinical Global Impression of Change (CGIC); Clinical Dementia Rating Sum of Boxes (CDR-SB); and Clinical Dementia Rating Global (CDR Global). Tests were performed by trained psychometrists with experience in administering these tests in clinical trials, who were blinded to whether the participants they were testing were in the treatment or the control group. Of these tests:
CGIC scores improved overall in the intervention group and worsened in the control group. Specifically, ten people in the intervention group showed improvement compared to none in the control group; none in the intervention group showed moderate worsening compared to three in the control group; and seven people in the intervention group were unchanged compared to eight in the control group.
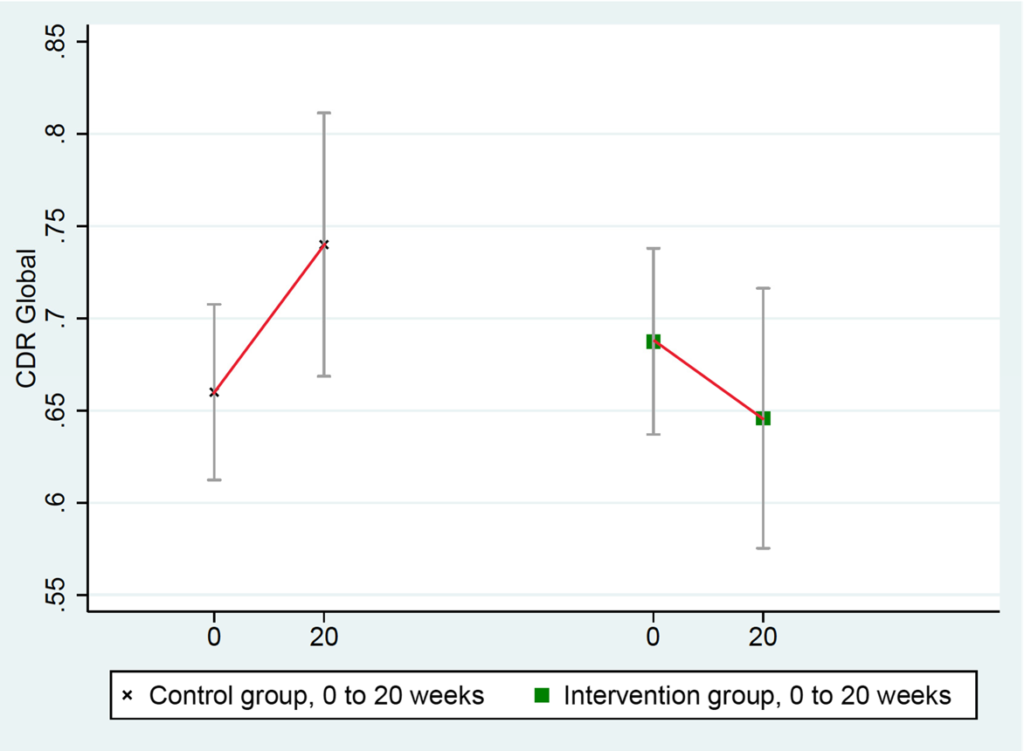
CDR-Global scores improved in the intervention group and worsened in the control group:
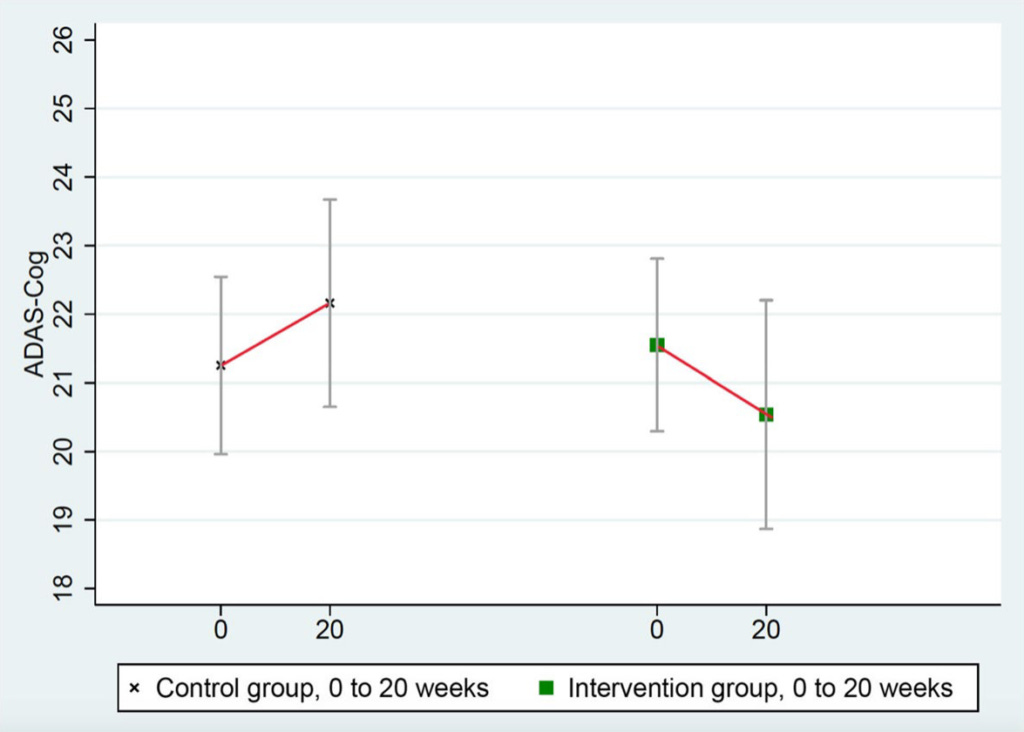
ADAS-Cog scores improved in the intervention group and worsened in the control group:
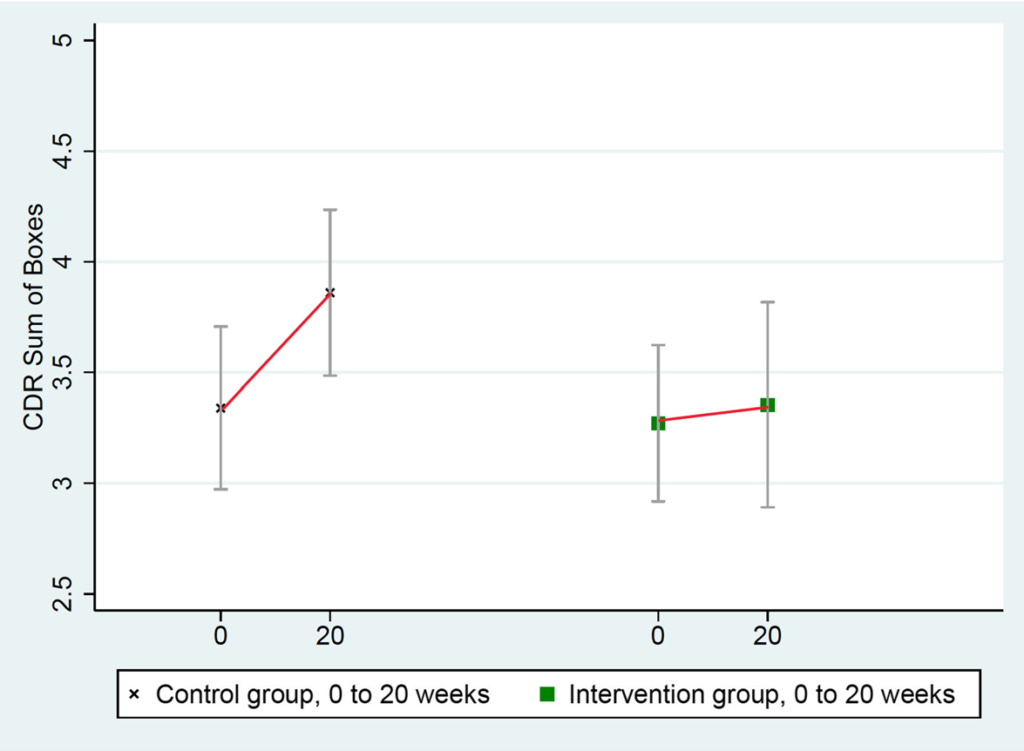
CDR-SB scores worsened significantly more in the control group than in the intervention group:
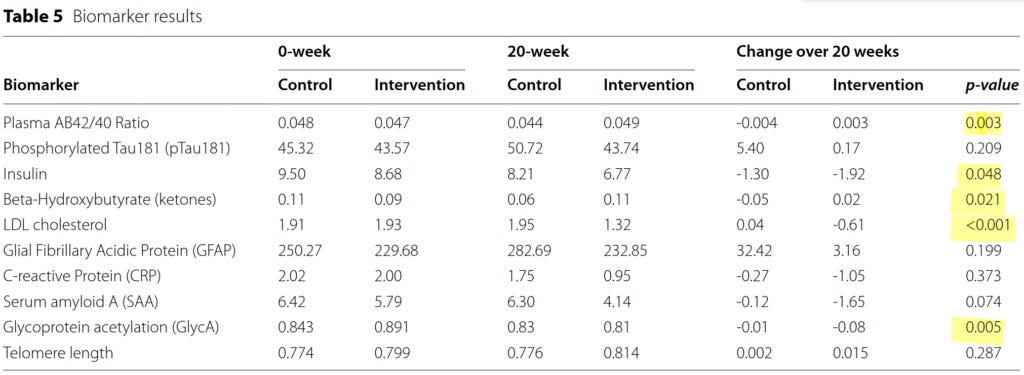
A range of biomarkers with an established role in the pathophysiology of Alzheimer's disease was tested:
The yellow highlights indicate biomarkers with a statistically significant difference in change from baseline to 20 weeks, between the intervention and control groups:
The plasma total β-amyloid (Aβ) Aβ42/40 ratio is now considered an important diagnostic and prognostic indicator of Alzheimer's disease, which strongly correlates with the presence of amyloid deposits in the brain on PET scan. The lower the plasma Aβ42/40 ratio, the greater the burden of amyloid in the cortex, the faster the accumulation of amyloid is occurring, and the steeper the pace of cognitive decline. The plasma Aβ42/40 ratio increased (i.e. improved) by 6.4 per cent in the intervention group but decreased by 8.3 per cent in the control group.
Fasting insulin levels declined in both groups, but more substantially in the intervention group. Haemoglobin A1c levels also declined in the intervention group. Both these biomarkers indicate improved insulin sensitivity. Loss of insulin sensitivity (i.e. insulin resistance) is a known driver of Alzheimer's disease.
Glycoprotein acetyls (GlycA) are inflammatory markers whose levels inversely correlate with lower global cognitive performance (i.e. the higher the GlycA, the worse the cognitive performance). GlycA increased in the control group while slightly decreasing in the intervention group.
LDL-cholesterol is positively associated with all measures of Alzheimer's disease neuropathology including neurofibrillary tangles and β-amyloid (i.e. the higher the LDL-C, the worse the brain pathology). LDL-C dropped by over 30 per cent in the intervention group in just 20 weeks (roughly equivalent to the effect of first-generation statins), but increased marginally in the control group.
β-Hydroxybutyrate is a ketone body that is utilised as a fuel source by neurons (brain cells), and can provide an alternative energy source to glucose in the case of insulin resistance, as well as reducing accumulation of β-amyloid and Tau proteins in the brain. β-Hydroxybutyrate levels declined in the control group but increased slightly in the intervention group.
Other biomarkers which moved in a beneficial direction in the intervention group compared to the control group, but did not reach statistical significance, included phosphorylated Tau181, glial fibrillary acidic protein, C‑reactive protein, serum amyloid A, and C-peptide. Telomere length (a marker of cellular age) increased in the intervention group and remained unchanged in the control group, but once again, the between-group difference did not reach statistical significance.
The gut microbiota of the intervention group underwent significant change, with an increase in several taxa of microorganisms that have previously been associated with reduced risk of Alzheimer's disease (including Blautia and Eubacterium), and a decrease in Prevotella and Turicibacter, which have previously been shown to increase with progression of Alzheimer's. No change was seen in the control group's microbiota composition.
It's worth noting that it's quite extraordinary that any difference at all was observable in many of these biomarkers of aging, brain pathology, general health and cognitive function over such a relatively short period - less than five months. Furthermore, demonstrating statistical significance in cognitive and biomarker changes with a small sample size is challenging. One of the reasons that drug trials typically recruit large numbers of patients is that the larger the sample size, the more likely you are to find some change in function or in a biomarker that's statistically significant, even if it's clinically meaningless. So did participants in the lifestyle intervention program experience clinically meaningful improvements in their daily function, or just statistically significant changes? Here's what two of them had to say:
How much more improvement might occur if participants maintained their new, healthy habits over the long term? Only time will tell, but on that note, statistical analysis revealed that the degree of adherence to the diet and lifestyle program correlated with the magnitude of improvement in cognitive test scores and biomarker levels. The more diligently participants stuck with the diet, supplements, exercise, stress management and group support, the better their results. The authors cautioned though, that quite a high degree of adherence was required:
"We also found that substantial lifestyle changes were required to stop the progression of MCI in these patients. In the primary analysis, this ranged from 71.4% adherence for ADAS-Cog to 95.6% adherence for CDR-Global to 120.6% adherence for CDR-SB. In other words, extensive lifestyle changes were required to stop or improve cognition and function in these patients. This helps to
explain why other studies of less-intensive lifestyle interventions may not have been sufficient to stop deterioration or improve cognition and function."
As exciting as the newly published research is, the study is bound to have its detractors. Previous studies that Dr Ornish conducted using a very similar diet and lifestyle program for the treatment of atherosclerotic cardiovascular disease and early-stage prostate cancer, were criticised because they included so many components that it was impossible to tell which ones "worked" and which were redundant. But this reductionist thinking exemplifies what's wrong with Western medicine, and why it's so catastrophically bad at dealing with pretty much anything other than serious bacterial infections and acute trauma.
Health is multifactorial. Disease is multifactorial.
If you want to recover from a chronic illness and restore vibrant health, you have to remove all the drivers of disease (or, at least, as many as you possibly can) and provide all the prerequisites for health. As he frequently recounts in his public lectures, Ornish first saw this when he was a medical student dealing with crippling depression. He devised and implemented his very first multicomponent intervention trial in the late 1970s, before he even graduated from medical school, and has been refining the program - both in terms of inclusions and delivery methods - ever since.
Each component of Ornish's early Alzheimer's reversal program has biological plausibility. I discussed the evidence that several of these components reduce the risk of dementia in my Preventing Dementia miniseries (Part 1; Part 2). Many more studies have been published since then that provide further support for the Ornish program:
Closer adherence to the Mediterranean-Dash Intervention for Neurodegenerative Delay diet (MIND) diet, a hybrid of the plant-rich Mediterranean diet and the pro-vegetarian Dietary Approaches to Stop Hypertension (DASH) diet, with specific focus on leafy green vegetables and berries, was shown to protect against dementia by slowing the pace of biological aging, in a study of 1644 participants aged 60 or over who were enrolled in the Framingham Offspring Cohort.
In a randomised clinical trial of a low-fat vegan diet very similar to the Ornish diet vs a portion-controlled diet, type 1 (insulin-dependent) diabetics assigned to the vegan diet reduced the amount of insulin they needed to take by 28 per cent and increased insulin sensitivity by 127 per cent, compared with participants assigned to the portion-controlled diet. Those assigned to the low-fat vegan diet were not told to restrict their carbohydrate intake or limit their portion size, and in fact added on average 111 g of carbohydrate per day to their pre-study intake, yet they still achieved vastly superior insulin sensitivity. They also lost on average 5.2 kg in 12 weeks compared with a nonsignificant change in body weight in the portion-controlled group, and their total insulin dose per kg of body weight dropped by 24 per cent, again with no significant change in the portion-controlled group.
Similarly, in the Stanford Twins Nutrition Study, healthy adult twins who were randomly assigned to a healthy vegan diet experienced significant decreases in low-density lipoprotein cholesterol (LDL-C) concentration, fasting insulin level, and body weight compared to their twin sibling who was assigned to a healthy omnivorous diet. Both diets were designed to be rich in fruits, vegetables, legumes, whole grains, nuts and seeds while limiting added sugars and refined grains, and all meals were supplied to participants for the first four weeks of the eight-week study.
In an 8-week randomised controlled trial, middle-aged men who undertook a treatment program incorporating a plant-centered phytonutrient-rich (but not vegan) diet, sleep optimisation, moderate exercise and relaxation guidance, and supplemental probiotics and phytonutrients, were found to reverse their biological age (as measured by the Horvath 2013 DNAmAge clock) by more than three years.
A single 10 minute bout of moderate-intensity running was found to increase local blood flow to the prefrontal cortex - the part of the brain that plays an important role in controlling mood and executive functions, and which is heavily involved in the memory deficits seen in people with Alzheimer's disease - and to improve performance on tests of executive function. A 30 minute walk, as recommended in the Ornish program, is a reasonable substitute for running, in older people.
Social isolation is associated with increased levels of two inflammatory markers - interleukin-6 (IL-6) and C-reactive protein (CRP) - which are both involved in the metabolic dysfunction and brain pathology that characterises Alzheimer's disease. The support group provided to the Ornish trial participants was expressly intended to mitigate the social isolation experienced by Alzheimer's patients and their spouses.
Is making - and sticking with - comprehensive diet and lifestyle changes to prevent and reverse Alzheimer's disease difficult? Undoubtedly, yes. Is it worth it? Watch those interviews with Ornish program participants once again, and decide for yourself.






wow Robyn ! a mind blowingly comprehensive report on the Ornish trial. This will be standard reading for all my clients and new clients. Enormous thanks for this magnificent effort .
Amazing! As someone who has recently experienced cognitive improvement as a result of comprehensive lifestyle measures, I can really relate to what the woman in the first video was saying - about feeling like your identity is coming back.
I love this statement: "If you want to recover from a chronic illness and restore vibrant health, you have to remove all the drivers of disease (or, at least, as many as you possibly can) and provide all the prerequisites for health".