GLP-1 agonists: Weight loss wonder-drugs, or disaster in the making?
Ozempic and Wegovy are taking the weight loss world by storm. Does the reality live up to the marketing hype?
A couple of months ago, I caught up with a cousin whom I hadn’t seen for several decades. I knew from conversations with other family members that she had been struggling with her weight since her 30s, and that she, like many other first and second degree relatives on both sides of my family, had been diagnosed with type 2 diabetes. But I was still unprepared for just how much larger she was than the last time I saw her, which just happened to be her wedding day. I felt intensely sad, as my mental picture of her as a svelte young woman in a bridal gown bumped up so jarringly against her current presentation.
She apologised for being late, explaining that she’d woken up feeling very ill, and had struggled to get out of bed. Then she told me why: she had been using Ozempic injections for the last several months. She had managed to lose around 15 kg, but at the cost of feeling nauseous and fatigued for a good chunk of every day.
My heart sank. I had read article after article on the dangers of GLP-1 agonists like Ozempic over the previous few months, and I wouldn’t wish these horrifying adverse effects on anyone. (OK, maybe on Dictator Dan Andrews or Scott 'Scummo' Morrison or Justin Turdeau or Klaus ‘Eat ze Bugs’ Schwab or any of the other Convid clowns and criminals, but let’s not go there.)
But I also knew, from reading the published medical literature, what would happen if my cousin stopped taking Ozempic: she would regain much, or even most, of the weight she’d lost, and the improvements she had made in her diabetic control would recede. What a terrible bind to find oneself in. And what a bonanza for Big pHarma - a drug that delivers the results that so many overweight people are desperate for, at a high price both in dollars and personal suffering, with rebound weight gain occurring as soon as it's stopped.
Since then, I’ve had several requests from readers to do a deep dive on Ozempic, Wegovy and related drugs. Well, there’s no time like the present, so here goes! In Part 1 of this series, I’ll discuss the basics: What exactly are these drugs, what conditions are they prescribed for, how do they 'work', and how good are they at what they're supposed to do?
Meet the GLP-1 agonists
The new kids on the weight loss drug block all belong to a class called glucagon-like peptide-1 (GLP-1) agonists, also known as GLP-1 receptor agonists, incretin mimetics, or GLP-1 analogs. They include:
Exenatide (Byetta, Bydureon)
Lixisenatide (Lyxumia, Adlyxin)
Liraglutide (Victoza, Saxenda)
Albiglutide (Eperzan, Tanzeum)
Dulaglutide (Trulicity)
Semaglutide (Ozempic, Wegovy)
GLP-1 agonists were originally approved to treat type 2 diabetics who do not achieve adequate blood glucose control on metformin, or cannot tolerate it.
Semaglutide and high-dose liraglutide subsequently received FDA approval as pharmacologic treatments for obesity, and to treat overweight patients with weight-related comorbidities.
In Australia, only liraglutide (Saxenda) is TGA-approved for weight loss, but off-label prescription of semaglutide (Ozempic) for weight loss has caused such a severe shortage that TGA has instructed doctors "not to initiate new patients on Ozempic for the time being unless there are no suitable alternatives or there is a compelling clinical reason to do so." Ozempic's manufacturer, Novo Nordisk, has warned that the supply shortage will persist throughout 2024.
(Side note: TGA laments that it's unable to address this shortage by stopping doctors from writing off-label prescriptions for Ozempic:
Funny how they magically summoned up the power to "regulate the clinical decisions of health professionals" and "prevent doctors from using their clinical judgement" when it came to prescribing ivermectin and hydroxychloroquine for the treatment of COVID-19, isn't it?)
What the heck is GLP-1, anyway?
To understand the mechanism of action of these drugs, you need to know about a couple of hormones involved in metabolism, blood glucose and appetite regulation:
Insulin is a hormone released by the pancreas in response to rising levels of glucose and certain amino acids in the bloodstream, after a meal. Insulin prompts cells to take up glucose, to burn as fuel or store for later use.
Glucagon-like peptide-1 (GLP-1) is a hormone that is made principally in the intestine, especially the final segment of the small intestine (the ileum) and the colon/large intestine. A small amount is produced in the poetically-named solitary tract of the brainstem. GLP-1 is secreted in response to the presence of nutrients from ingested food in the gut. GLP-1 is one of a group of gut peptides called incretins. (Peptides are short chains of amino acids, the building blocks of protein.) Incretins are released after eating, and their function is to potentiate the release of insulin.
Aside from its role in promoting insulin release, GLP-1 also:
Slows down gastric emptying, the rate at which the stomach discharges its contents into the small intestine;
Increases satiety, the feeling of fullness after a meal which discourages further eating;
Decreases appetite centrally (i.e. within the brain), by decreasing the reward value of food - especially high-fat, high-calorie food;
Inhibits apoptosis ('cell suicide') of insulin-producing beta cells, thus preserving the capacity of the pancreas to produce insulin
Increases natriuresis and diuresis, the excretion of sodium and water via urine;
Decreases inflammation; and
Has protective effects on the heart (for example, improving coronary blood flow, cardiac output, and endothelial function) and nervous system.
GLP-1 is released in two phases. The first burst occurs within 10-15 minutes after food intake, and is believed to be triggered by neuroendocrine factors (i.e. hormones that are secreted in response to nervous system signalling). The second phase occurs 30 to 60 minutes after a meal, and is influenced by the arrival of nutrients in the ileum and colon. Not all nutrients are created equal, when it comes to stimulating GLP-1 secretion. More on that, in Part 4 of this mini-series.
To exert its activity, GLP-1 binds to receptors on the membranes of target cells. GLP-1 receptor agonist drugs work by binding to these GLP-1 receptors, essentially tricking the receptors into 'thinking' that they are being activated by genuine, honest-to-god GLP-1. That's why these drugs are called "incretin mimetics" - they mimic the effects of a biochemical that the body naturally produces and uses to regulate its metabolic activities.
Importantly though, this mimicry is only partial. For starters, endogenous (self-produced) GLP-1 exerts its activities via both endocrine and neuronal routes - that is, GLP-1 is not only released as a hormone, which has to diffuse into the bloodstream to reach its target cells, but it also influences the activities of neurons in the gut, the brain, and the vagus nerve which connects the two. Conversely, GLP-1 agonist drugs only exert their effects through the endocrine route.
In part because of this difference between GLP-1 and the drugs that mimic it, the drugs are designed to remain in circulation for considerably longer than endogenous GLP-1. While the half-life of GLP-1 is less than two minutes (meaning that within two minutes after its release, the concentration of the hormone has already dropped by half), liraglutide has a half-life of 13 hours and semaglutide, approximately seven days. What could possibly go wrong with prolonging the activity of a biochemical that's supposed to hang around for only a couple of minutes, for an entire week? More on that in Part 2.
Before I briefly review some of the key clinical trials on GLP-1 agonists for weight loss, I want to bring your attention to an incredibly important but easy-to-miss point: These drugs promote weight loss whilst increasing circulating levels of insulin.
As I mentioned in Ketogenic diets: Part 3 – Weight loss, many proponents of ketogenic diets insist that insulin is a “fat-making” hormone, and that very low carbohydrate diets cause weight loss, regardless of caloric intake, by reducing insulin secretion. In that previous article, I discussed many studies that disprove the carbohydrate-insulin hypothesis of weight gain. The efficacy of insulin-raising GLP-1 agonists as weight loss agents should be the final death-blow to this untenable hypothesis... but one thing I've learnt about many low carbers (especially the loudest ones) is that they don't like to let facts get in the way of a good story.
How well do GLP-1 agonists work for weight loss?
A systematic review and meta-analysis of 25 randomised controlled trials of exenatide and liraglutide found that participants taking either of these GLP-1 agonists lost an average of 2.9 kg (6.4 lb) more than participants taking placebo. Non-diabetics lost more weight on GLP-1 agonists than diabetics (3.2 kg vs 2.8 kg more than controls, respectively). Control groups received either placebo, insulin, or oral antidiabetic drugs: third generation sulphonylurea compounds, dipeptidyl peptidase 4 inhibitors, thiazolidinediones, or metformin. Participants given the highest doses of GLP-1 agonists had greater weight loss.
Just in case you blinked and missed it, the only GLP-1 agonist approved as a weight loss agent in Australia, liraglutide (Saxenda), causes a stupendous 2.9 kg more weight loss than placebo. Not very impressive, eh?
But what about semaglutide, the drug whose demand has massively outstripped supply because an aggressive social media marketing campaign triggered an avalanche of off-label prescriptions for weight loss?
Semaglutide (Ozempic, Wegovy) boasts more impressive weight loss stats than exenatide or liraglutide. In a systematic review and meta-analysis of four randomised controlled trials, enrolling a total of 3613 obese, non-diabetic individuals, participants assigned to semaglutide lost on average 11.85 per cent more of their body weight than controls. A hypothetical obese individual weighing 100 kg could thus expect to lose almost 12 kg more from taking semaglutide, than from taking a placebo.
That sounds pretty awesome. It's important to point out though, that all four trials included in this meta-analysis were paid for by Novo Nordisk, the Danish pharma behemoth that produces semaglutide. Here are the author lists, conflict of interest and funding statements from each trial:
1. Once-Weekly Semaglutide in Adults with Overweight or Obesity
2. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial
3. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial
4. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial
I've highlighted in blue the names of authors who are credited on more than one of these four pivotal studies, to point out the overlapping authorship. The pink highlights are there to draw your attention to the role played by medical communication companies such as Spirit Medical Communications Group and ArticulateScience. And the yellow highlights are just for laughs at their absurdity, in the context of a laundry list of conflicts of interest.
In their disturbing and insightful book, The Illusion of Evidence-Based Medicine, Jon Jureidini and Leemon McHenry explain the nature and roles of each of the cogs in the vast machine of the medical-industrial complex - a vast machine designed for a singular purpose: to get as many men, women and children on as many drugs as possible, for as long as possible:
"In response to Marcia Angell's (2000) editorial 'Is Academic Medicine for Sale?' in the New England Journal of Medicine, Thomas J. Ruane responded with the quip: 'No. The current owner is very happy with it.' The pharmaceutical industry largely controls academic medicine by engaging academic physicians as third-party promotional agents for their products...
Pharmaceutical companies have gained unprecedented control over the testing of their own drugs... The companies select for publication the trials that show the drugs have passed a minimal test, often using a flawed design that favors their drugs, and then conceal those trials with negative results. Since the companies have intellectual property rights to the data they generate, they control the dissemination of information. Prescribing physicians are then exposed to a distorted profile of drugs. The companies hire contract research organizations (CROs) to conduct the clinical trials, academic researchers to design the trials and act as clinical investigators, medical communications companies to ghostwrite the publications [my emphasis], and public relations firms to create positive images of the drugs for prescribing physicians and the public. All are eager to serve their client's best interest, irrespective of whether the drugs being evaluated are the latest miracles of science. Almost always, they are not."
The Illusion of Evidence-Based Medicine, pp. 8-9
Take another look at the funding statements, overlapping author lists, and credits to medical communications companies, and ask yourself, 'How high should be my level of trust in the published results of semaglutide trials?'
When we dig into those study results, more concerns emerge. Firstly, these trials are not nearly large enough to establish the drug's safety profile. The number of participants ranges from 611 to 1961, and in the largest trial, one third of participants received a placebo injection. Such small studies cannot possibly detect rare side effects, which by definition affect between 1 in 10,000 and 1 in 1000 people, and would even miss many uncommon side effects (those that strike between 1 in 1000 and 1 in 100 people).
Secondly, it's very evident that semaglutide only works as long as you keep taking it. Look at this figure, taken from the third study mentioned above:
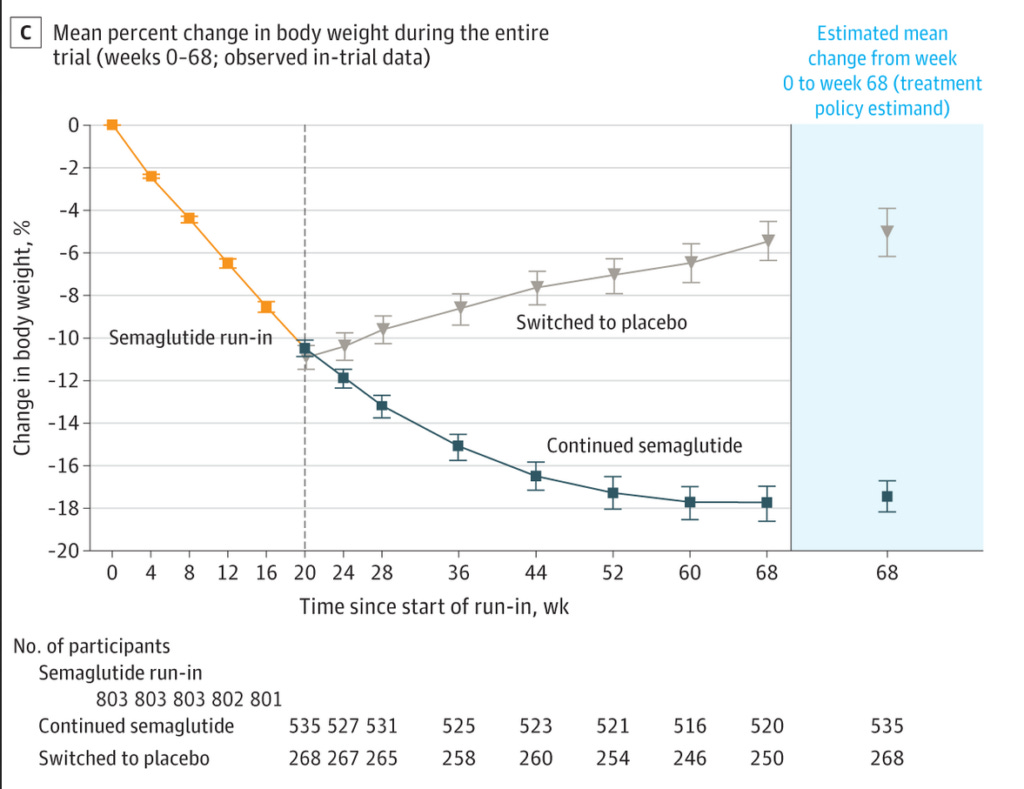
In this trial, all participants took a 2.4 mg injection of semaglutide once per week for 20 weeks, and then one third of them were switched to placebo for an additional 48 weeks, whilst the other two thirds continued on weekly treatment with semaglutide. As you can see, those who were switched to placebo immediately began to regain the weight they had lost, although they still ended the trial below their starting weight.
On a related note, the third major area of concern is that it's quite apparent that the effectiveness of semaglutide wanes over time. Compare these figures from three of the above-mentioned trials:
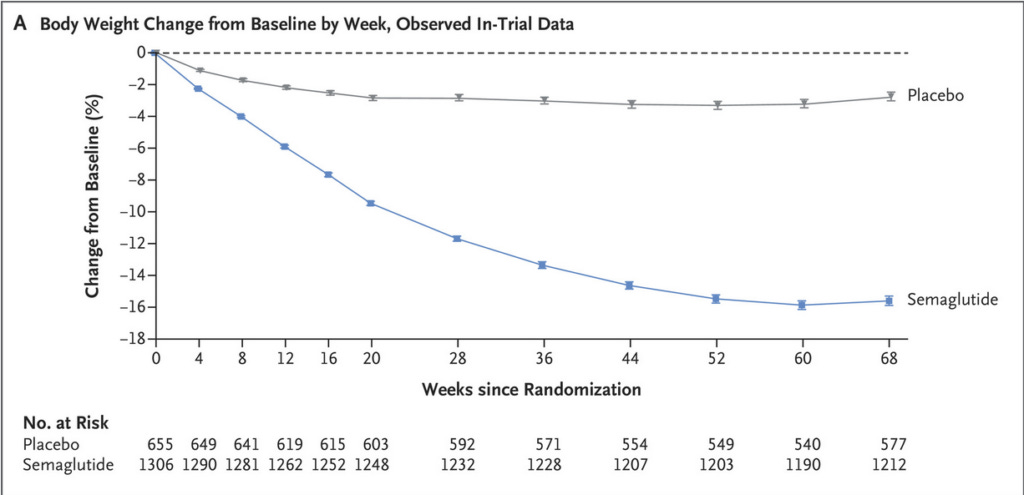
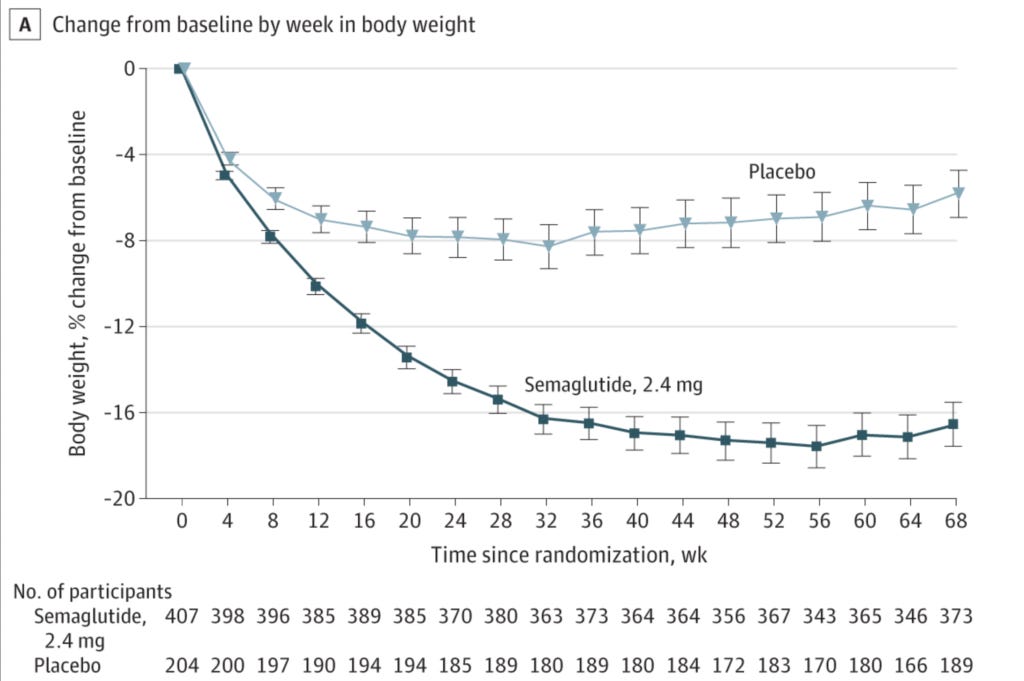

Notice how each of these trials ran for 68 weeks, and that weight loss was either plateauing or reversing by this point? Why did these three trials run for precisely 68 weeks each? According to the clinical trial record for the Step 1 trial (from which the first of these three figures was taken), the date of participants' last contact with the trial site was week 75, so what happened to participants after the magical 68 weeks was up? Did the weight loss graphs start to look not quite so good between weeks 68 and 75? And was that because many participants were stopping their medication due to side effects? So many questions!!!!
Speaking of side effects, these four trials did report on many of them, and more have emerged since GLP-1 agonists hit the mass market. But we'll get to that in Part 2!
This post has taken well in excess of 30 hours to research and write. If you feel you’ve obtained value from reading it, please consider a paid subscription:















Do we know what kind of weight loss ? i.e. muscle vs fat ? Is it injected because an oral version would be even crappier ? Looking forward to Pt 2, I've heard there is something called 'ozempic face' which perhaps implies muscle loss. I just took a look on reddit re. Ozempic, it's very topical.
Thank you, thank you for taking the time to research and write this article. I have a staff member who I am trying to wake up to the Pharma control of medicine. Unfortunately she is not listening and started on these drugs last year. I have so many patients taking them as well and the weight loss they have experienced has blinded them to any possibility that there could be long term side effects from signing up to be part of this experiment.