Unpacking the ‘ketogenic diet cures cancer’ myth: Part 2
Continuing my analysis of distortions and misrepresentations in Dr Thomas Seyfried's interview on Diary of a CEO.
In last week's post, I dissected the claims made by Boston College professor of biochemistry, Dr Thomas Seyfried, in the first 22 minutes of his interview on the Diary of a CEO podcast.
I've skipped over a fairly lengthy section of the interview because it contains multiple repetitions of Seyfried's claim that cancer is driven by loss of mitochondrial function in tumour cells, and the resultant metabolic switch to “fermentation” (aerobic glycolysis), which I comprehensively analysed in Part 1.
Let's pick up at around 31 minutes into the interview, where Seyfried begins to lay out his ideas about how to prevent the development of cancer.
31:10 "You can avoid that [i.e. carcinogen-induced damage to mitochondria that induces them to switch from producing energy via oxidative phosphorylation to carrying out aerobic glycosis], that's why I'm saying, if you can keep your mitochondria healthy... so even if you are exposed to chemical carcinogens, even if you are exposed to all these things but you're keeping your body as healthy as you possibly can, you could possibly delay or even prevent the damage to the mitochondria even though you have the, even though you are being exposed to this, so it's a it's actually in your hands, you can actually reduce risk for cancer by knowing what keeps your mitochondria healthy."
I devoted much of Part 1 to critiquing Seyfried's claims that the metabolic switch from oxidising food-derived fuel molecules in the mitochondria, to carrying out aerobic glycolysis (which he describes as "fermentation") in the cytosol (the fluid inside the cell), is the fundamental driver of cancer. Hopefully, you'll remember from Part 1 that not only do up to 40 per cent of tumours not make the switch to glycolysis, but that even those that do, retain the function of their mitochondria, and can efficiently switch between using glucose, multiple amino acids (not just glutamine), fatty acids, ketone bodies and short chain fatty acids as fuels, depending on availability, environmental conditions and the stage of tumour growth.
However, since I wrote Part 1, I stumbled across a study that cuts Seyfried's hypothesis off at the knees. In 2021, researchers demonstrated that tumour cells steal mitochondria from adjacent cells, using a straw-like structure called a nanotube. The phenomenon was first observed in an in vitro (petri dish) experiment, in which a field-emission scanning electron microscope (FESEM) captured these astonishing images of breast cancer cells extending nanotubes into adjacent T cells (a type of immune system cell which seeks out and destroys cancer cells) in order to extract their mitochondria, which were labelled with a dye so that their passage between the cells could be tracked:
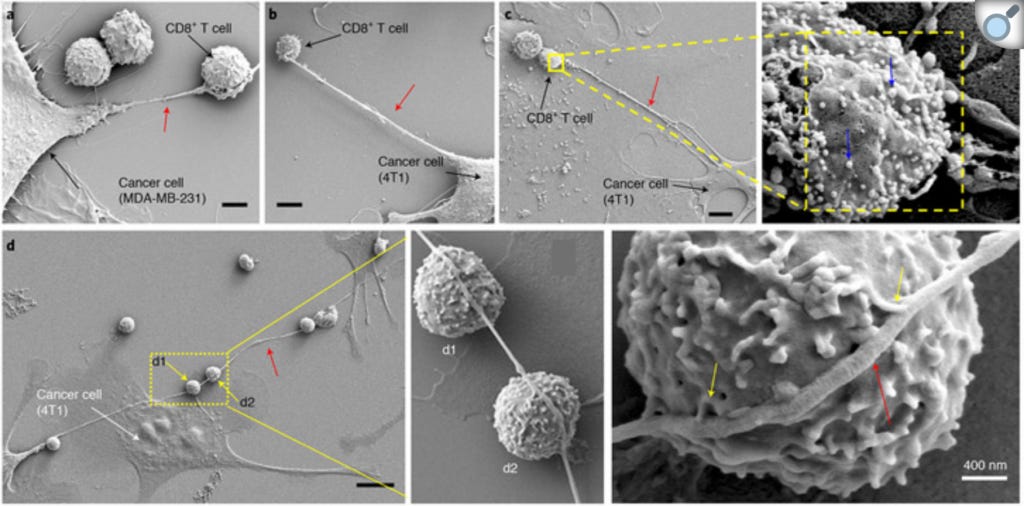
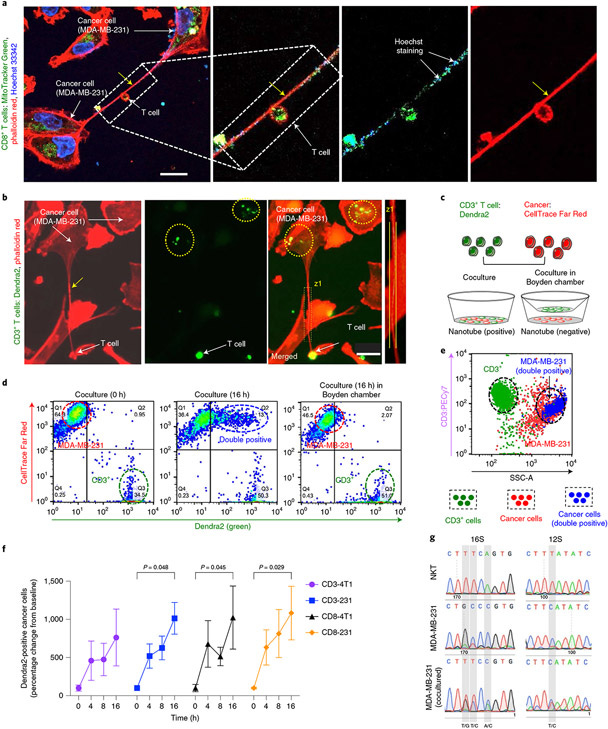
The researchers then measured the metabolism of both types of cells, and found that the cancer cells that had stolen mitochondria from the T cells exhibited significantly greater energy production, while the mitochondria-depleted T cells suffered a significant reduction in energy production. Furthermore, the thieving cancer cells proliferated at a much faster rate over the next several days, indicating that they were using the hijacked mitochondria - not glycolysis - to fuel their accelerated growth.
Then the researchers leveraged the fact that mitochondria have their own genome, which is distinct from the cellular genome located in the nucleus, to trace the trafficking of T cell mitochondria into cancer cells derived from tumour samples from many different types of cancer, including lung, pancreatic, colorectal, and breast. Not only did they find that cancer cells with a higher capacity to steal mitochondria had a faster cell division rate, meaning that they proliferated more aggressively, but people whose tumours excelled at trafficking mitochondria from T cells had a shorter life expectancy than those with less capacity.
What all this means, is that "keeping your mitochondria healthy" will do you no damn good if you have a tumour that's skilled in the art of hijacking mitochondria from T cells. All that those "healthy mitochondria" will do, is to supercharge the growth of your mitochondria-thieving tumour. Bummer, huh?
35:16: "The biggest devastating information against the somatic mutation theory [the theory that mutations in the cell's genome initiate the cancer process] is if you take the nucleus from a tumour cell, cleanly take it out of the tumour cell, and you have another normal cell here, you take the nucleus out of the normal cell and you put the tumour cell into that cytoplasm, you get regulated growth, no dysregulated growth. But if I have the normal cell and have a tumour cell, take the tumour nucleus out of there and take the normal nucleus and put it into the tumour cytoplasm which contains mitochondria, defective mitochondria - dysregulated cell growth. This has been seen over and over and over again.
[Interviewer] So just to summarise that - so if you take the tumour nucleus out of the cell and put it into a normal healthy cell...
Yes.
[Interviewer] Everything's fine.
Everything is fine.
[Interviewer] But if you take a healthy cell nucleus and put it into a tumour cell, you still have the same...
Dysregulated cell growth - tumour growth.
[Interviewer] So - which means that it's not the nucleus.
Absolutely!
[Interviewer] It's something else.
It's something else, and that's the mitochondria."
The series of experiments described above, in which tumour cells hoovered up mitochondria from T cells in order to power their own proliferation, pretty much deep-sixes Seyfried's claim. Whatever factors in the tumour cytoplasm may be playing a role in whether a cell does or does not become cancerous, it's pretty clear that it's not the mitochondria, since tumour cells are perfectly capable of hijacking mitochondria from completely healthy cells in order to fulfill their nefarious purposes.
And there's yet another piece of evidence against Seyfried's contention that cells turn cancerous because their mitochondria become defective, forcing them to switch to aerobic glycolysis for energy production, which I have discovered since writing Part 1. Since Otto Warburg made his famous observation, researchers have been trying to figure out why cancer cells would resort to using the inefficient process of glycolysis to produce energy, rather than oxidative phosphorylation, even when there's plenty of oxygen and fully-capable mitochondria available.
In a 2021 study, researchers found that aerobic glycolysis helps cells to regenerate large quantities of a molecule called nicotinamide adenine dinucleotide (NAD+), which cells need in order to synthesise DNA and other molecules. Rapidly-growing cells need NAD+ more than they need ATP - that is, they need the 'raw materials' that are required for them to make copies of themselves, more than they need the concentrated energy source provided by ATP. Oxidative phosphorylation is highly efficient at generating ATP, but if cells accumulate more ATP than they can use, respiration slows and production of NAD+ also slows, which means that cells don't have enough NAD+ to continue their rapid proliferation.
As the authors explain,
"This argues that cells engage in aerobic glycolysis when the demand for NAD+ is in excess of the demand for ATP."
Increased demand for NAD+ relative to ATP drives aerobic glycolysis
Or, in plain English, a cell that is rapidly proliferating will deliberately turn on aerobic glycolysis in order to meet its need for NAD+. Interestingly, certain non-cancer cells engage in the exact same process when they need to proliferate rapidly. Lymphocytes 'turn on' aerobic glycolysis when they are engaging in clonal expansion in order to mount an immune response, and fibroblasts do the same when they have to proliferate rapidly in order to heal wounds.
In summary, aerobic glycolysis is not something that cancer cells resort to in desperation, because their mitochondria are too defective to generate ATP through oxidative phosphorylation. It's something they do 'on purpose' in order to facilitate their rapid proliferation. So one would expect to find the highest rates of aerobic glycolysis in the most aggressive tumour cells, because they're the ones that need the most NAD+.
36:17 "You have to be a hopeless ideologue to think that cancer is a genetic disease. It's a silent assumption in the field that cancer is a genetic... every textbook of biology, cell biology and bio [says] cancer is a genetic disease.
[Interviewer] Why hasn't people's opinions changed, despite the evidence that you present?
It's a very difficult thing. It goes back to when you have one theory replacing another theory. It's called paradigm shifts and in all history of science, paradigm shifts have been met with great, great resistance."
39:55 "We get 7 billion dollars a year for cancer research in the National Cancer Institute, awarding many, many - not all - many grants to look for gene mutations and all this kind of stuff, and we have drugs that are extremely expensive based on a somatic mutation theory of cancer that are basically not dropping the death rate."
40:32 "When you look at the scientific advisory committee of all these societies that you're running for [i.e. charity runs for cancer research], they all think... publish papers on cancer as a genetic disease. It's too hard for the field to accept at this point; it's too traumatic, what I'm saying. It's too disruptive to a massive industry at this time. They will come to gradually adjust to what I'm saying; it's just a matter of time because we cannot continue this trajectory."
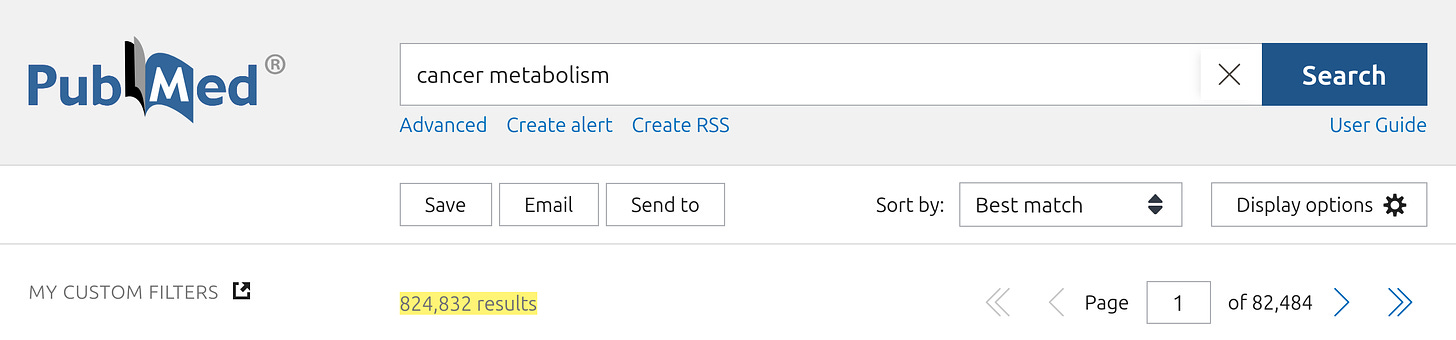
Seyfried's insistence that cancer research is stubbornly clinging onto the somatic mutation theory of cancer as its dominant paradigm, and neglecting the study of the metabolism of cancer cells which he believes is the key to understanding, preventing and overcoming the disease, is belied by the fact that over 800,000 articles have been published in the scientific literature on the subject:
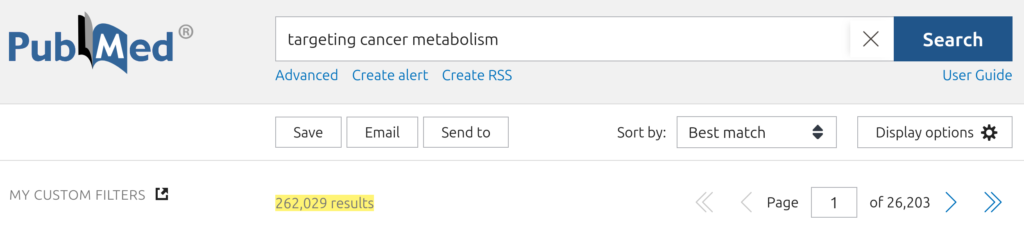
... with over 200,000 devoted to the targeting of cancer metabolism as a therapeutic strategy:
Every single one of those studies had to have a funding source, and most of that funding comes from grants - including those issued by the National Cancer Institute. The other major funding source is pharmaceutical companies and biotech start-ups, which - far from being traumatised by the idea that the somatic mutation theory may not be the be-all and end-all of oncogenesis - are positively wetting themselves over the opportunity to hit paydirt by developing cancer treatments that target cancer metabolism. Unlike classic chemotherapy agents, which are generally administered in an intensive but relatively short course, drugs that target the metabolism of cancer cells would likely be administered for the rest of the patient's life. Ka-ching, ka-ching!
Seyfried is not some lonely, prescient prophet preaching his metabolic gospel in the wilderness, while the pharma pharisees stop their ears and myopically focus on mutated genes. Cancer researchers have been investigating the metabolic abnormalities exhibited by cancer cells, and exploring ways of targeting these abnormalities in order to treat cancer, for decades, with the number of studies published annually on this topic accelerating dramatically since the early 2000s:
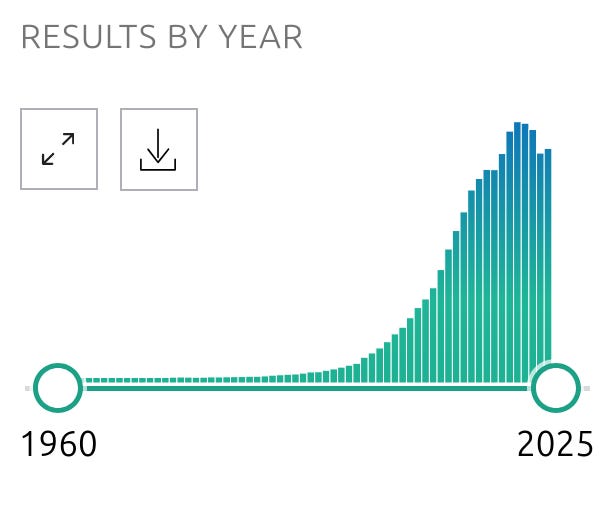
38:19 "The mitochondria is the centre of the problem with cancer - not the nucleus, the mitochondria. It's a mitochondrial metabolic disease, and once you realise that, we're going to drop these death rates massively in a very, in a number of years, for sure."
Not to flog a dead horse on this, but if cancer cells can not only make efficient use of their own mitochondria for energy generation, but can also hijack healthy cells' mitochondria to supercharge their own proliferation, it's a bit hard to imagine how Seyfried's 'paradigm shift' is supposed to drastically reduce cancer death rates.
53:53 "People say to me, what should I eat? Should I eat this and that? Normally you would [i.e. should] eat foods that have very low glycaemic index, which means the speed with which glucose is released. Like a banana [is] very high in glycemic index. You eat a banana, your blood sugar immediately spikes. Many fruits are like that."
Wrong. Bananas have a low to moderate glycaemic index, ranging from 45 (low) for unripe bananas, through to 60 (moderate) for ripe fruit. But more importantly, bananas have a low to moderate glycaemic load (again depending on ripeness), which is a far more accurate measure of how a given food impacts on blood sugar level than glycaemic index, because it takes into account the amount of carbohydrate contained in the food. In fact, the vast majority of fruits have a low glycaemic index and a very low glycaemic load, with the exception being dried fruits. I don't know what prevents a biology professor from looking up a glycaemic index chart to fact-check himself; motivated reasoning, perhaps?
1:10:06 "Can you believe this? I can't even believe I'm saying this stuff myself. You really got to know the biochemistry, and you have to know the physiology of your own body, and you have to understand evolutionary biology."
I agree, Dr Seyfried. I can't believe you're saying this stuff, when I - without the benefit of a PhD in biology - can go online and find evidence that pretty much everything you said in this interview about biochemistry, physiology and evolutionary biology is either completely wrong, or a partial truth.
1:13:18 "Fasting is a powerful way to get your body into nutritional ketosis but it ain't easy. Try doing it; you try doing it - see how easy - it ain't easy, right? But that's why we developed this procedure... a zero carb diet for 14 days; 10 to 14 days. Just zero carb, eat meat, fish, chicken, whatever you want, but just don't eat any bread, pasta this kind of thing."
As I mentioned in Part 1, fasting-induced ketosis and nutritional ketosis are not the same thing. Fasting induces higher blood levels of ketone bodies than the ketogenic diet, and is more effective at promoting autophagy (the process of removing and recycling damaged components of cells, which promotes cellular rejuvenation).
And Seyfried's advice to consume a diet composed exclusively of animal-derived foods to reduce one's risk of cancer, while avoiding all carbohydrate-rich foods, is frankly preposterous. Red meat consumption is associated with an increased overall cancer incidence; both processed and red meat intake increase the risk of lung cancer, breast cancer, and colorectal cancer. A systematic review and dose-response meta-analysis of prospective cohort studies found that low-carbohydrate diets are associated with a 14 per cent higher risk of dying of cancer, while if the low-carbohydrate diet is animal-based, that risk nudges up to 16 per cent higher. Participants in the National Health and Nutrition Examination Survey (NHANES; 1999–2010) who were in the lowest quartile of carbohydrate intake had 36 per cent higher cancer mortality.
Conversely, consumption of fruits, vegetables, breakfast cereal (definitely not on my list of recommended foods, but there you go) and fibre (which is found only in the plant foods that Seyfried advises people to avoid) is protective against lung cancer. High fruit and vegetable consumption is associated with reduced risk of breast cancer, including both hormone receptor-positive and -negative. Bread and breakfast cereals were both found to reduce the risk of colorectal cancer. Multiple studies have found that a higher intake of fruits, vegetables and fibre reduce the risk of a number of different cancer types. Women who reduced their dietary fat intake by 8–10 per cent of daily calories, and increased their carbohydrate intake by the same percentage, largely by eating more vegetables, fruit, and grains, reduced their risk of dying of breast cancer by 16 per cent. Men with prostate cancer have lower plasma concentrations of nutrients found only in plants - lutein, lycopene, α-carotene and β-carotene - and higher concentrations of nutrients which characterise high animal product intake, including calcium, sulphur and iron. Cancer patients who had a higher intake of fruits and vegetables before diagnosis have a lower risk of dying, with the protective effect particularly evident for ovarian and head and neck cancers.
In start contrast, there is not a single study that has found that the animal-based low-carbohydrate diet that Seyfried promotes, reduces the risk of developing cancer. Not one.
(By the way, wanna know what happens to Maasai men - one of those peoples "who live according to their traditional ways" that Seyfried waxes lyrical about, when they attend olpul, a bush retreat in which they consume nothing but meat and medicinal herbs for four weeks? Before I tell you what happens to them, you should know that when they're eating their regular diet, the average distribution of energy intake is 67 per cent carbohydrate, 24 per cent fat and 9 per cent protein. That's not a low-carb diet. During olpul, that energy distribution is 5 per cent carbohydrate, 55 per cent fat and 40 per cent protein. And what happens to them? Half of these super-fit, lean men develop disorders in glucose metabolism while eating their practically all-meat diet.)
So if Seyfried is wrong about so many things, does that mean that his metabolic approach to treating cancer has no value?
No, not at all! Even if Seyfried is wrong about how and why ketogenic diets affect cancer cell metabolism, the therapy might still work in some cases. In fact, based on my review of the literature, I think there is value in his approach for certain forms of cancer - in particular, some types of brain tumour. It's no coincidence that the case studies that Seyfried cites most frequently, are patients with glioblastoma multiforme (GBM), an absolutely hideous type of brain cancer with a median survival time for adults of just 14.6 months, and a five year survival rate of 6.8 per cent (although the longest documented survivor of this wretched disease has been kicking on for over 25 years post-diagnosis).
Out of all the different types of cancer which afflict human beings, brain tumours are the most consistent in exhibiting the Warburg effect... although, as I wrote in Ketogenic diets: Part 5 – Cancer:
"Not all glioma patients will respond positively to a ketogenic diet, because some glioma cells express ketolytic enzymes and hence can use ketone bodies as a fuel. As the researchers concluded, 'Our results showing that malignant gliomas have differential expression of ketolytic and glycolytic enzymes are consistent with previous studies that have shown that these are genetically heterogeneous tumors.'"
Several case studies of the use of ketogenic diet for brain tumours have been published:
Two young girls with astrocytomas were put on a ketogenic diet, one after her tumour had failed to respond to radiotherapy and chemotherapy, and the other while receiving chemotherapy. PET scans showed a 22 per cent decrease in glucose uptake at the tumour site, and both girls were still alive at the time of publication of the case study, five and four years after diagnosis, respectively.
In a case series of six patients who underwent a ketogenic diet during treatment for GBM, two died and four were still alive at a median follow-up of 14 months (which is just under the median survival time anyway). Patients were reported as tolerating the diet well.
20 patients with recurrent glioblastoma (i.e. GBM that had been treated, but grew back) were put on a ketogenic diet alone or in combination with bevacizumab (Avastin). Three patients had to quit the diet because they could not tolerate it. One patient achieved a minor response (i.e. the tumour shrank a little) while two had stable disease (i.e. the tumour did not grow) after six weeks. Median survival from enrollment was 32 weeks.
A pilot study examined the effects of a ketogenic diet on quality of life in 16 patients with advanced metastatic cancer of various types (none of them brain tumours), who had run out of conventional therapeutic options. Of these,
"One patient did not tolerate the diet and dropped out within 3 days. Among those who tolerated the diet, two patients died early, one stopped after 2 weeks due to personal reasons, one felt unable to stick to the diet after 4 weeks, one stopped after 6 and two stopped after 7 and 8 weeks due to progress of the disease, one had to discontinue after 6 weeks to resume chemotherapy and five completed the 3 month intervention period. These five and the one who resumed chemotherapy after 6 weeks report an improved emotional functioning and less insomnia, while several other parameters of quality of life remained stable or worsened, reflecting their very advanced disease."
So in terms of quality of life, what we can learn from this study is that less than half the patients could tolerate the diet. Of those who did stick to it, as demonstrated by their urinary excretion of ketone bodies, half suffered tumour progression while the tumours of the other half stopped growing. Hardly a ringing endorsement of the ketogenic diet as a cancer therapy, although to be fair, these were such late-stage cases that even slowing down tumour growth is something to be celebrated.
A safety and feasibility trial tested the ketogenic diet in 17 patients with advanced cancer of various types (including two patients with brain cancer), of whom 11 were evaluable. Again, none were on conventional treatment as previous treatments had failed. After four weeks on the ketogenic diet, five of the 11 patients had progressed (i.e. their tumours had grown) while six had stable or partially improved disease. Only three patients continued the ketogenic diet past 16 weeks and of these, two developed brain metastases.
Contrary to Seyfried's claim that cancer researchers refuse to consider targeting tumour metabolism as a treatment strategy, there are numerous clinical trials underway to assess the efficacy of ketogenic diets in cancer therapy. So within the next few years, you'll have some more solid evidence to inform your choices, if you ever find yourself in the unenviable position of facing a cancer diagnosis. It may well be the case that certain tumours are able to be kept in check by some version of a ketogenic diet, perhaps augmented by periodic water-only fasting or a fasting-mimicking diet.
It's a shame that Dr Thomas Seyfried undermines his credibility by spouting so much absolute nonsense, because it may turn out that his metabolic therapy for cancer is truly helpful for some patients. But it's just plain wrong to claim that all cancer cells demonstrate the Warburg effect (they don't), that being in permanent ketosis is the healthy and natural state of humans because we evolved on a ketogenic diet (it isn't, and we didn't), and that you can prevent cancer by putting yourself into nutritional ketosis (you can't).
In closing, I'll reiterate the advice that I gave at the end of Part 1: Question everything you see, hear and read! The field of health and nutrition is chock-full of people spruiking their opinions. Never uncritically accept anything they say. Thanks to the power of the internet, it's dead easy to type a question such as 'Do all cancer cells use glycolysis for energy production?' into a search engine, and it doesn’t take a 150 IQ to sort evidence-based articles from blogs written by self-promoting nincompoops, so there's no excuse for not doing your own basic fact-checking. And although I urge you to use your own brainpower to read the results that that search returns - because if you don't use it, you'll lose it - you can always utilise a built-in AI tool, like the Leo AI assistant built into the Brave browser, to quickly parse highly technical and jargon-filled articles.
And finally, this post has taken me approximately 20 hours to research and write. It takes much more time to debunk statements that aren’t true, than it does to spout them! I make all my posts freely available to all readers, because I believe we all deserve access to information that helps us to take greater control over our health. But I rely on my small core of paid subscribers to provide this service to those who genuinely can’t afford it.




Sample size of one, but I personally know someone who had stage 4 cancer who went into remission with a ketogenic diet. Thanks for bursting my bubble that it may not be the cure for all types of cancer for every individual! Haha. But I guess of course it’s going to be more complex than that
So, it seems to me that keeping your mitochondria healthy is likely to reduce your chances of developing cancer, unless you have the unfortunate phenotype that is able to steal mitochondria from other cell types, in which case the healthier your mitochondria, the quicker the cancer will grow. But not all people have cancers who can do that, and having healthy mitochondria will reduce your risk of a zillion other diseases and make you feel better, so it’s generally a good thing to try and do, IMO.
I think your naturopathic bias towards loving fruits and veggies has biased you a little too, in this article. I don’t think you can take much from nutritional studies about cancer risk etc because of the inherent confounders in those type of studies. I think it ultimately depends on your bioindividuality and what combinations of food you eat. For instance, if you eat a lot of carbs/sugars AND a lot of fat, things go a bit pear shaped. If you do mostly one or the other, things tend to go better.
Even if Ketogenic and carnivore diets are truly likely to increase cancer risk (which I think is debatable), they probably do so if the person eats a lot of processed meat, or cooks it at too high a heat. But these diets are really helpful for many other diseases, so they still have benefit for many individuals.
I’m pretty convinced by the science of those diets, though my palette personally struggles with the idea of not having some fresh fruits and vegetables.