The strange tale of hydroxychloroquine
Queensland's Chief Health Office has quietly revoked the previous CHO's threat to jail doctors who prescribe hydroxychloroquine for COVID-19. And the official reason doesn't make any sense.
On 12 March 2022, the Chief Health Office (CHO) of Queensland, Dr John Gerrard, revoked a directive issued on 7 April 2020 by previous CHO and now governor of Queensland, Dr Jeanette Young, which imposed criminal sanctions – a fine of up to $13 785 or 6 months in prison – on doctors and pharmacists who prescribed or dispensed hydroxychloroquine for prevention or treatment of COVID-19.
Here’s the original directive issued by Young, in the very early days of the manufactured COVID crisis:
And here is Gerrard’s revocation of that directive:
The revocation was published with little fanfare; a sharp-eyed subscriber to my Substack brought it to my attention on the morning of 6 April (thanks, Timothy!), and that evening, the Murdoch-owned Courier Mail ran an article on it.
Apparently, like Voldemort, hydroxychloroquine gains power from merely having its name spoken: The Courier Mail could not bring itself to use the word “hydroxychloroquine” in the headline or lede, euphemising it as “a certain drug” and then – misleadingly, since it is routinely prescribed for a host of conditions besides malaria – “an antimalarial drug”:
Jodie Munro O’Brien, the Courier Mail ‘journalist’ (I use the term loosely, for reasons that will become evident) tasked with reporting the story, simply reiterated the official government narrative that the ban on prescribing hydroxychloroquine for COVID-19 had been instituted because of fears that off-label use for this purpose would lead to “a supply shortage, leaving those already prescribed the drug for other, unrelated ailments without their medicine.”
If you’re wondering, “Why didn’t the government look for ways to increase the supply of hydroxychloroquine, rather than threatening doctors and pharmacists with six months in the slammer or a fine of nearly 14 grand for making it available to sick people?” then you’re thinking like a journalist. Perhaps you could apply for Ms Munro O’Brien’s job… or perhaps not, since the legacy media prefers stenographers to actual journalists.
And indeed, the hydroxychloroquine supply problem was promptly solved by Queensland businessman Clive Palmer, who bought 40 million doses of the drug from overseas sources by late April 2020 (i.e. just a few weeks after Jeanette Young issued the ban), and donated more than 22 million of them to the national medical stockpile before being informed by a Commonwealth Health Department spokeswoman on 18 June 2020 that “no further donations were required”.
However, despite these facts having been reported in the very newspaper which employs her, Ms Munro O’Brien simply regurgitated the Queensland Health spokeswoman’s illogical explanation for Gerrard’s action:
“A Queensland Health spokeswoman said the revocation of the ban was because there was no longer a supply shortage threat, but the medication was still not approved for Covid-19 treatment.”
Restrictions that threatened doctors with a $13000 fine if they prescribed a certain drug revoked
Say what? The “supply shortage threat” was solved almost two years ago. And since off-label prescription of drugs is perfectly legal and universally practised (accounting for up to 40% of prescriptions for adults and up to 90% in some hospitalised paediatric patients), no “approval” is required for doctors to prescribe hydroxychloroquine for prevention or treatment of COVID-19.
Jeanette Young’s criminalisation of the use of hydroxychloroquine to treat COVID-19 patients was just one of a Series of Unfortunate Events that befell this 65+ year old drug that is included in the World Health Organisation’s Model List of Essential Medicines, in the weeks before and in the months after that body declared that COVID-19 was a “Public Health Emergency of International Concern” (PHEIC, which I presume is pronounced like “fake”) on 30 January 2020.
Mathew Crawford has covered the many twists and turns of this gothic tale in The Chloroquine Wars section of his must-read ‘Rounding the Earth’ Substack, but here is a chronology of some of the most significant events:
On 15 January 2020 – two weeks before WHO declared its PHEIC for COVID-19 – the French medical health agency rescinded its 2008 authorisation of over-the-counter sales of more than 200 commonly-used drugs, including hydroxychloroquine, in community pharmacies. The French government’s restrictions on prescription of the drug escalated over the ensuing months, prompting the formation of a doctors’ collective calling themselves “Let Doctors Prescribe” to advocate for the restoration of physicians’ freedom to exercise their clinical judgment. A representative of the collective, Dr Violette Guérin, complained in late March 2020 that
“Today there is an escalation of directives aimed at restricting [hydroxychloroquine’s] use from the Directorate General of Health. And it remains authorized in the setting where it is the least effective, that is to say in the intensive care units.”
“We want to self-prescribe chloroquine”, asks a group of doctors
On 11 March 2020, Dr John Gerrard treated actor Tom Hanks and his wife Rita Wilson for COVID-19 at Gold Coast University Hospital. No details of the treatment they received have been issued, due to patient confidentiality. However, a retrospective observational cohort study of the first 197 COVID‐19 patients managed by Gold Coast Hospital and Health Service, between February and April 2020, coauthored by Gerrard, clearly shows that chloroquine drugs were in use at the time Gerrard treated Hanks and Wilson: “Twenty‐one patients received hydroxychloroquine (mean duration of therapy 4.4 days), one patient received chloroquine (5 days).” 63 patients were hospitalised (54 for medical reasons and the remainder for “observation”, “public health reasons” and “social reasons”), 5 required intensive care unit admission and 3 required intubation; no patients died. No concerns were expressed in this paper about any toxicity of chloroquine drugs.
On 24 March 2020, the Therapeutic Goods Administration (TGA) announced that GPs and specialists except those practising dermatology, intensive care medicine, paediatrics and child health, physician and emergency medicine, were no longer permitted to prescribe hydroxychloroquine, citing “demand shortages” created by use of the drug for treatment of COVID-19 that “pose… a serious health risk to individuals currently using this medication”. Note that TGA did not raise any safety concerns about the off-label use of hydroxychloroquine to treat COVID-19, merely putative supply issues.
Also on 24 March 2020, the governor of New York, Andrew Cuomo, issued an executive order barring pharmacists from dispensing “hydroxychloroquine or chloroquine except when written as prescribed for an FDA-approved indication; or as part of a state approved clinical trial related to COVID-19 for a patient who has tested positive for COVID-19, with such test result documented as part of the prescription. No other experimental or prophylactic use shall be permitted, and any permitted prescription is limited to one fourteen day prescription with no refills”. In other words, with one stroke of his pen, Cuomo cancelled out physicians’ right to prescribe chloroquine drugs off-label and prevented people who were already taking these drugs for FDA-approved purposes from obtaining a sufficient supply to last them through the 100-day shutdown that he imposed four days earlier, on 20 March.
Then on 28 March 2020, the governors of the US states of Michigan and Nevada issued executive orders prohibiting physicians from prescribing, and pharmacists from dispensing, “hydroxychloroquine and chloroquine to patients for the treatment of COVID-19 outside of a hospital”. Both orders cited the same justification as Jeanette Young – the concern that hoarding of the drug by people afraid of contracting SARS-CoV-2 infection would threaten its availability to people taking it for “a legitimate medical purpose” – thus arrogating to themselves and their bureaucrats, most of whom are not licensed medical professionals, the right to decide what constitutes “legitimate medical purposes”. Furthermore, restricting the drug to hospitalised patients ensured that it could not be used in the early treatment of COVID-19, when its benefits are the most pronounced.
On the same day, 28 March 2020, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) permitting “emergency use of oral formulations of chloroquine phosphate (CQ) and hydroxychloroquine sulfate (HCQ) to be distributed from the Strategic National Stockpile (SNS)” (my emphasis) for use in treating people with COVID-19. This implies that there were perfectly adequate reserves of hydroxychloroquine for such purposes, which would have spared the supply available in community pharmacies, thus invalidating the concerns with supply issues that the governors of Michigan and Nevada invoked as the rationale for their executive orders.
On 22 May 2020, one of the world’s most prestigious medical journals, The Lancet, published a study which claimed to draw on a multinational registry comprising 671 hospitals located in six continents. Analysing data supplied by the Surgical Outcomes Collaborative (managed by Surgisphere Corporation), which supposedly included over 96 000 patients hospitalised for COVID-19 between 20 December 2019 and 14 April 2020, the authors concluded that patients treated with chloroquine or hydroxychloroquine (alone or in combination with a macrolide antibiotic, such as azithromycin), had between a 33% and 45% higher chance of dying than those whose treatment did not include a chloroquine drug, as well as up to triple the risk of developing a potentially lethal type of abnormal heart rhythm called ventricular arrhythmia.
There was just one small problem with the Lancet study – the dataset was completely fake. Six days after its publication, on 28 May 2020, more than 120 researchers and medical professionals published an open letter addressed to the study’s authors, and Lancet editor Richard Horton, raising serious concerns about the integrity of the data source, study methodology and statistical analysis.
And on 5 June 2020, Lancet issued a retraction notice, in which the study’s authors claimed that Surgisphere had denied them access to the full dataset for the purposes of independent peer review. No wonder, since the dataset simply did not exist. It subsequently emerged that Surgisphere – a company which had claimed to run one of the largest and fastest hospital databases in the world – had only a handful of employees, few of them with any background in data analysis or scientific background; their “science editor” was actually a science fiction author and fantasy artist (which seems oddly appropriate) while their marketing executive worked as an adult model and events hostess. Apparently, neither the authors of the Lancet study (led by cardiologist Mandeep Mehra, who is a professor at Harvard Medical School as well as a director at Boston’s Brigham & Women’s Hospital) nor the journal’s editorial and peer review team, were capable of discovering these facts for themselves. I’m sure the fact that Mehra’s employer, Brigham & Women’s Hospital, was also running two clinical trials for the drug remdesivir, sponsored by the $1000-per-pill drug’s manufacturer, Gilead, had absolutely nothing to do with Mehra’s lapse in judgement.However, despite the retraction, the damage to hydroxychloroquine had already been done. On 26 May 2020, the WHO paused recruitment to the hydroxychloroquine arm of its global randomised clinical trial to identify effective treatments for COVID-19, SOLIDARITY, citing the Lancet study as justification. The UK and French regulatory bodies followed suit, halting their national trials of hydroxychloroquine. And while WHO resumed the hydroxychloroquine arm of SOLIDARITY on 3 June (two days before the Lancet retraction), recruitment into these trials had become difficult due to the bad publicity.
On 5 June 2020, the chief investigators of the UK Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial, which received funding from the Bill and Melinda Gates Foundation and the Wellcome Trust, reported that they had called an early halt to the hydroxychloroquine arm of the study due to interim analysis of data showing “no beneficial effect of hydroxychloroquine in patients hospitalised with COVID-19”. This interim analysis was posted on the preprint server medRxiv on 15 July 2020 and published in the New England Journal of Medicine (NEJM) on 19 November 2020.
The NEJM article concluded that COVID-19 patients given hydroxychloroquine were 9% less likely to be discharged from the hospital alive within 28 days than those who did not receive the drug, and 14% more likely to require mechanical ventilation after hospitalisation.
And on reading the details of the treatment and dosage schedule, these dismal results are no surprise. The trial was not just set up to fail, it was arguably designed to kill patients.
Firstly, treatment began a median of 9 days after symptom onset. Hydroxychloroquine works by inhibiting viral replication, which peaks in the first few days after symptom onset and ceases by around the eight day after onset of symptoms of COVID-19. In other words, by the time patients enrolled in the RECOVERY trial received hydroxychloroquine, it was too late for the drug to provide any clinical benefit to them.
Secondly, those randomised to receive hydroxychloroquine were given 800 mg of the drug at baseline, another 800 mg 6 hours later, followed by 400 mg every 12 hours for the next 9 days or until discharge, whichever occurred earlier. In the Supplementary Appendix, the authors justify this dosage schedule as follows:
“The hydroxychloroquine dose regimen was based on previous pharmacokinetic modelling of plasma and whole blood hydroxychloroquine concentrations in healthy volunteers, the treatment of malaria and in rheumatological conditions. The choice of dose and predicted safety margins were also informed by pharmacometric studies of chloroquine in the treatment of both severe and uncomplicated malaria and in self-poisoning.”
But as David Jayne, professor of clinical autoimmunity at Cambridge University, pointed out, this dosage schedule does not remotely resemble those used for either malaria or rheumatological disease, and substantially exceeds the known toxic dose of hydroxychloroquine. In an article in the British Medical Journal (BMJ), Jayne shared his concerns:
“Current recommended doses for rheumatologic disease are typically 300-400 mg/day and… the maximum dose for malaria has been 800 mg in the first 24 hours. ‘The reasons behind the dose selection in the RECOVERY trial are unclear,’ he says.
‘Hydroxychloroquine overdose is associated with cardiovascular, neurological, and other toxicities, occurring with doses over 1500 mg, and higher doses are associated with fatality.’ He is concerned that hydroxychloroquine toxicity may have contributed to the adverse outcomes and that conclusions based on these results may be unreliable.”
On 15 June 2020, FDA revoked its EUA for chloroquine and hydroxychloroquine. It cited the early termination of the hydroxychloroquine arm of the RECOVERY trial as one of its principal reasons for doing so, but the press release announcing the revocation revealed that some of the mud flung by the discredited Surgisphere data-that-wasn’t had stuck: “Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
On 19 June 2020, the hydroxychloroquine arm of WHO’s SOLIDARITY trial was “discontinued for futility“, although WHO only announced that it was terminating the hydroxychloroquine trial on 4 July 2020, on the grounds that the drug produced “little or no reduction in the mortality of hospitalized COVID-19 patients when compared to standard of care.” In fact, the interim results, published in the NEJM, were that hydroxychloroquine increased the risk of death by 19%.
Once again, hydroxychloroquine was administered too late to show benefit (most patients had already been in hospital for two or more days before treatment commenced) and a toxic dose was administered: 800 mg at baseline, another 800 mg after 6 hours, then 400 mg every 12 hours for 10 days. In other words, patients received 2000 mg of hydroxychloroquine in the first 24 hours of treatment – well in excess of the dose of 1500 mg that is known to be potentially fatal.
And WHO had been alerted that its hydroxychloroquine dosage schedule was hazardous back in May 2020 by the Indian Council of Medical Research, which warned that the dose being used in international trials was four times higher than that specified in the protocol set by the Indian government to treat severe COVID-19 patients requiring ICU management.
Low-income nations continued to utilise hydroxychloroquine for pre-exposure prophylaxis and early treatment, with considerable success:
However, in rich Western countries including Australia, the drug’s name was now mud. Other clinical trials which included hydroxychloroquine treatment arms were amended to ditch the drug, including the Australasian COVID-19 Trial (ASCOT) for which Dr John Gerrard was the Gold Coast University Hospital contact.
The National COVID-19 Clinical Evidence Task Force continues to recommend against the use of hydroxychloroquine in any circumstances. It states that “evidence indicates that hydroxychloroquine is potentially harmful and no more effective than standard care in treating patients with COVID-19”, citing the RECOVERY and SOLIDARITY trials as providing the “majority of evidence” for its position without acknowledging the fact that these trials used the drug too late for it to be effective, and employed a known toxic dose.
An open letter sent to Jeanette Young on 29 September 2020 by Federal MPs George Christensen and Craig Kelly, pointing out the flaws in the RECOVERY trial and directing Young to the growing body of evidence supporting the appropriate use of hydroxychloroquine, was met by deafening silence (except by ABC “fact checkers” whose intrepid investigation of the controversy consisted of quoting a TGA statement and asking the executive director of the National COVID-19 Clinical Evidence Task Force, Julian Elliott, for his opinion on the MPs’ claims).
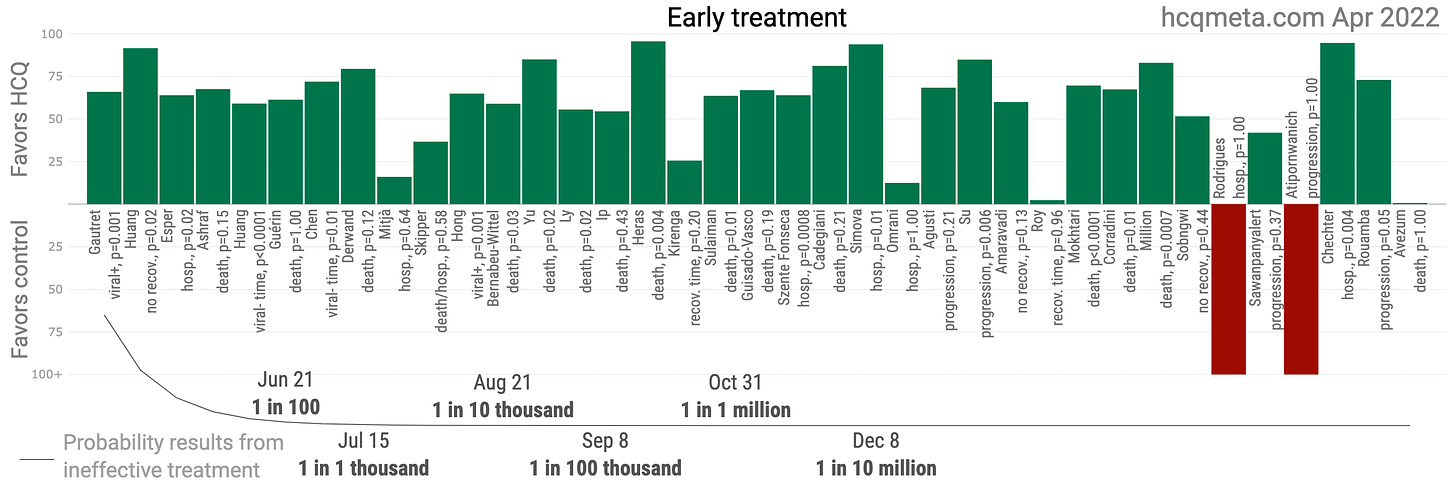
Young extended the hydroxychloroquine ban, which had been due to expire on 2 October 2020. By this time, the probability that hydroxychloroquine was ineffective for early treatment of COVID-19 was nearing 1 in 1 million:
After considering these facts, several important questions arise:
Why did multiple jurisdictions in rich countries impose restrictions on doctors’ ability to use hydroxychloroquine for pre-exposure prophylaxis and early treatment of COVID-19 in outpatient settings, all at around the same time, and all citing the same demonstrably false rationale (the concern that supply of the drug to people who were already taking it for non-COVID-related conditions would be compromised, when ample amounts were already in national stockpiles, or were quickly obtained)? Unless one is a coincidence theorist, this coordination hints at an organised hit job on hydroxycholoroquine, a cheap generic that is one of the world’s most widely prescribed drugs, with a long history of safe use, even in pregnancy and breastfeeding.
Why did the RECOVERY and SOLIDARITY trials use a dosage schedule of hydroxychloroquine that is known to be toxic and potentially fatal? And why did they restrict the use of the drug to advanced stages of COVID-19 when their own clinical trial protocols made it clear that the researchers understood that its mechanism of action as an antiviral was no longer relevant once patients had passed the viral replication stage?
What does Queensland’s Chief Health Officer, John Gerrard, know about the use of hydroxychloroquine for treatment of COVID-19? He presided over the infectious diseases department of Gold Coast University Hospital during the first wave of infections in early 2020, when chloroquine drugs were used to treat more than 10% of COVID-19 patients. What are the real reasons for his revocation of Jeanette Young’s directive, given that the rationale proffered by the Queensland Health spokeswoman – that there were no longer any supply concerns – is demonstrably false, the supply problem having been solved nearly two years prior (and indeed, 5 months before Young renewed the directive)?
And finally, why have doctors simply acquiesced to the dramatic erosion of their right to practise medicine, including the right to prescribe TGA-approved medicines off-label if the scientific literature provides evidence of potential benefit – which it very clearly does, in the case of properly-prescribed hydroxychloroquine?
I guess the Courier Mail‘s hard-hitting journalist, Jodie Munro O’Brien, will be working tirelessly to get us answers to all these questions, any day now.










“The Science” giveth and “The Science” taketh away. By “The Science” we shall live, for “The Science” is our great protector, our great savior, to “The Science” we pledge our allegiance and support, regardless the cost.
-The book of Fauci.
Australians who worked in PNG took chloroquine to prevent malaria. Dr J Young now Lt Governor in Qld (jobs for the girls) was a failed GP.